Abstract
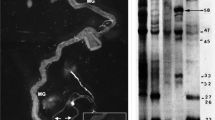
A homologue of the chaperonin protein of the HSP60 family has not been shown so far inDrosophila. Using an antibody specific to HSP60 family protein in Western blotting and immunocytochemistry, we showed that a 64-kDa polypeptide, homologous to the HSP60, is constitutively present in all tissues ofDrosophila melanogaster throughout the life cycle from the freshly laid egg to all embryonic, larval and adult stages. A 64-kDa polypeptide reacting with the same antibody in Western blots is present in all species ofDrosophila examined. Using Western blotting in conjunction with35S-methionine labeling of newly synthesized proteins and immuno-precipitation of the labeled proteins with HSP60-specific antibody, it was shown that synthesis of the 64-kDa homologue of HSP60 is appreciably increased by heat shock only in the Malpighian tubules, which are already known to lack the common HSPs.
Similar content being viewed by others
References
Schlesinger, M. J., Ashburner, M., and Tissieres, A., Heat Shock Proteins: From Bacteria to Man. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY 1982.
Lindquist, S., A. Rev. Biochem.55 (1986) 1151.
Morimoto, R., Tissieres, A., and Georgopoulos, C., in: Stress Proteins in Biology and Medicine, p. 1, Eds. R. I. Morimoto, A. Tissieres and C. Georgopoulos. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 1990.
Ritossa, F. M., Experientia18 (1962) 571.
Tissieres, A., Mitchell, H. K., and Tracy, U., J. molec. Biol.84 (1974) 389.
Nover, L., Heat Shock Response of Eukaryotic Cells. Springer-Verlag, Berlin 1984.
McMullin, T. W., and Hallberg, R. L., Mol. cell. Biol.8 (1988) 371.
Hendrick, J. P., and Hartl, F.-U., A. Rev. Biochem.62 (1993) 349.
Lakhotia, S. C., and Singh, A. K., J. Genet.68 (1989) 129.
Wessing, A., and Eichelberg, D., in: The Genetics and Biology ofDrosophila, p. 1. Eds M. Ashburner and T. R. F. Wright. Academic Press, London 1978.
Lakhotia, S. C., and Mukherjee, T., Chromosoma81 (1980) 125.
Singh, A. K., and Lakhotia, S. C., Devl Genet.9 (1988) 193.
Lakhotia, S. C., and Singh, B. N., Indian J. expl Biol.31 (1993) 301.
Miller, S. G., Leclerc, R. F., and Erdos, G. W., J. mol. Biol.214 (1990) 407.
Singh, B. N., and Lakhotia, S. C., Curr. Sci.69 (1995) 178.
Harlow, E., and Lane, D., Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 1988.
Laemmli, U. K., Nature227 (1970) 680.
Patel, N. H., Meth. Cell Biol.44 (1994) 445.
Ellis, R. J., and Van der Vies, S. M., A. Rev. Biochem.60 (1991) 321.
Gething, M.-J., and Sambrook, J., Nature355 (1992) 33.
Hemmingsen, S. M., Woolford, C., Van der Vies, S. M., Tilly, K., Dennis, D. T., Georgopoulos, C. P., Hendrix, R. W., and Ellis, R. J., Nature333 (1988) 330.
Goloubinoff, P., Gatenby, A. A., and Lorimer, G. H., Nature337 (1989) 44.
Braig, K., Otwinowski, Z., Hegde, R., Boisvert, D. C., Joachimiak, A., Horwich, A. L., and Sigler, P. B., Nature371 (1994) 578.
Rusanganwa, E., and Gupta, R. S., Gene126 (1993) 67.
Ursic, D., and Genetzky, B., Gene68 (1988) 267.
Ursic, D., and Culbertson, M. R., Mol. cell. Biol.11 (1991) 2629.
Kubota, H., Hynes, G., Carne, A., Ashwerth, A., and Willison, K., Curr. Biol.4 (1994) 89.
Gupta, R. S., Biochem. International20 (1990) 833.
Hemmingsen, S. M., Nature357 (1992) 650.
Frydman, J., Nimmesgern, E., Erdjument-Bromage, H., Wall, J. S., Tempst, P., and Hartl, F.-U.. EMBO J.11 (1992) 4767.
Ursic, D., and Culbertson, M. R., Nature356 (1992) 392.
Willison, K. R., and Kubota, H., in: The Biology of Heat Shock Proteins and Molecular Chaperones, p. 309. Eds R. I. Morimoto, A. Tissieres and C. Georgopoulos. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY 1994.
Nath, B. B., and Lakhotia, S. C., Genome32 (1989) 676.
Tiwari, P. K., Mohan, D. R. K., and Joshi, A., J. Biosci.20 (1995) 341.
Lakhotia, S. C., Proc. DAE Symp. Adv. Molec. Biol., p. 199. Lib. & Inf. Service, BARC, Bombay 1990.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lakhotia, S.C., Singh, B.N. Synthesis of a ubiquitously present new HSP60 family protein is enhanced by heat shock only in the Malpighian tubules ofDrosophila . Experientia 52, 751–756 (1996). https://doi.org/10.1007/BF01923984
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01923984