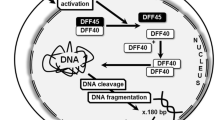
Abstract
Recent research has focused on identifying the biochemical events associated with the apoptotic process. These include specific degradation of the chromatin which was described by Wyllie in 1980 [1], with the report of the appearance of discretely sized DNA fragments from apoptotic rat thymocytes. The fragments corresponded in size to strands of DNA that were cleaved at internucleosomal regions and create a ‘ladder patterns’ when electrophoresed on an agarose gel. Because of its near universality, internucleosomal DNA degradation is considered a diagnostic hallmark of cells undergoing apoptosis. It is of great interest to identify the enzymes involved, and some of the candidates will be discussed.
Similar content being viewed by others
Literatur
Wyllie A. (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous nuclease activation. Nature284: 555–556
Arends M. and Wyllie A. (1991) Apoptosis: mechanisms and roles in pathology. Int. Rev. Expl. Path32: 223–251
Kerr J., Wyllie A. and Currie A. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer26: 239–257
Wyllie A., Kerr J. and Currie A. (1980) Cell death: the significance of apoptosis. Int. Rev. Cytol.68: 251–306.
Cohen J. and Duke R. (1984) Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J. Immunol.132: 38–42
Wyllie A., Morris R., Smith A. and Dunlop D. (1984) Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J. Path.142: 67–77
Arends M., Morris R. and Wyllie A. (1990) Apoptosis: the role of the endonuclease. Am. J. Path.136: 593–608
Compton M. and Cidlowski J. (1992) Thymocyte apoptosis: a model of programmed cell death. Trends Endocr. Metab.3: 17–23
Schwartzman R. and Cidlowski J. (1993) Mechanism of tissue-specific induction of internucleosomal deoxyribonucleic acid cleavage activity and apoptosis by glucocorticoids. Endocrinology133: 591–599
Oberhammer F., Fritsch G., Pavelka M., Froschl G., Tiefenbacher R., Purchio T. and Schulte-Hermann R. (1992) Induction of apoptosis in cultured hepatocytes and in the regressing liver by transforming growth factor-bl occurs without activation of an endonuclease. Toxic. Lett.64/65: 701–704
Ucker D., Obermiller P., Eckhart W., Apgar J., Berger N. and Meyers J. (1992). Genome digestion is a dispensible consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol. Cell. Biol.12: 3060–3069
Filipski J., Leblanc J., Youdale T., Sikorska M. and Walker P. (1990) Periodicity of DNA folding in higher order chromatin structures. EMBO J.9: 1319–1327
Compton M. and Cidlowski J. (1986) Rapid in vivo effects of glucocorticoids on the intergrity of rat lymphocyte genomic DNA. Endocrinology118: 39–45
Duke R., Chernenak R. and Cohen J. (1983) Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc. Natl Acad. Sci. USA80: 6361–6365
Hallick R., Chelm B., Gray, P. and Orozco E. (1977) Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nuc. Acids Res.4: 3055–3064
Dowd D., MacDonald P., Komm B., Haussler M. and Miesfeld R. (1992) Stable expression of the calbindin-D28K complementary DNA interferes with the apoptotic pathway in lymphocytes. Mol. Endocrinol.6: 1843–1848
Clawson G., Norbeck L., Hatem C., Rhodes C., Amiri P., McKerrow J., Patierno S. and Fiskum G. (1992) Ca2+-regulated serine protease associated with the nuclear scaffold. Cell Growth Diff.3: 827–838
Smyth M. Browne K., Thia K., Apostolidis V., Kershaw M. and Trapani J. (1994) Hypothesis: cytotoxic lymphocyte granule serine proteases active target cell endonucleases to trigger apoptosis. Clin. Expl. Pharmacol. Physiol.21: 67–70
Brown D., Sun X.-M. and Cohen G. (1993) Dexamethasone-induced apoptosis involves cleavage of DNA to large fragments prior to internucleosomal fragmentation. J. Biol. Chem.268: 3027–3039
Bortner C. and Cidlowski J. (1996) The role of volume regulation in apoptosis. Am. J. Physiology (in press)
Peitsch M., Polzar B., Stephan H., Crompton I. and MacDonald H. (1993). Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J.12: 371–377
Barry M. and Eastman A. (1993) Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Archs Biochem. Biophys.300: 440–450
Tanuma S.-I. and Shiokawa D. (1994) Multiple forms of nuclear deoxyribonuclease in rat thymocytes. Biochem. Biophys. Res. Commun.203: 789–797
Gaido M. and Cidlowski J. (1991) Identification, purification and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. J. Biol. Chem.266: 18580–18585
McConkey D., Hatzell P., Nicotera P. and Orrenius S. (1989) Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J.3: 1843–1849
Ribeiro J. and Carson D. (1993) Ca2+/Mg2+-dependent endonuclease from human spleen: purification, properties, and role in apoptosis. Biochemistry32: 9129–9136
Zhivotovsky B., Nicotera P., Bellomo G., Hanson K. and Orrenius S. (1993) Ca2+ and endonuclease activation in radiation-induced lymphoid cell death. Expl Cell Res.207: 163–170
Miyauchi K., Ogawa M., Shibata T., Matsuda K., Mori T., Ito, K., Minamiura N. and Yamamoto T. (1986) Development of a radioimmunoassay for human deoxyribonuclease I. Clin. Chim. Acta154: 115–123
Eastman A. (1994) Deoxyribonuclease II in apoptosis and the significance of intracellular acidification. Cell growth Diff.1: 7–11
Compton M. and Cidlowski J. (1987) Identification of a glucocorticoid-induced nuclease in thymocytes. J. Biol. Chem.262: 8288–8292
Rosenthal A. and Lacks S. (1977) Nuclease detection in SDS-polyacrylamide gel electrophoresis. Analyt. Biochem.80: 76–90
Cleveland D., Fischer S., Kirschner M. and Laemmli U. (1977) Peptide mapping by limited proteolysid in sodium dodecyl sulfate and analysis by gel elecrophoresis. J. Biol. Chem.252: 1102–1106
Caron-Leslie L. and Cidlowski J. (1991) Similar actions of glucocorticoids and calcium on the regulation of apoptosis in S49 cells. Mol. Endocrinol.5: 1169–1179
Caron-Leslie L. and Cidlowski J. (1994) Apoptosis: signal transduction and modes of activation. In: Ovarian Cell Interactions: Genes to Physiology, pp. 1–22. New York, Springer-Verlag
Montague J., Gaido M., Frye C. and Cidlowski J. (1994) A calcium-dependent nuclease from apoptotic rat thymocytes is homologous with cyclophilin. J. Biol. Chem.269: 18877–18880
Handschumacher R., Harding M., Rice J. and Drugge R. (1984) Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science226: 544–546
Koletsky A., Harding M. and Handschumacher R. (1986) Cyclophilin: distribution and variant properties in normal and noeplastic tissues. J. Immunol.137: 1054–1059
Ryffel B., Woerly G., Greiner B., Haendler B., Mihatsch M. and Foxwell B. (1991) Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology72: 399–404
Dawson T., Steiner J., Lyons W., Fotuhi M., Blue M. and Snyder S. (1994) The immunophilins, FK506 binding protein and cyclophilin, are discretely localized in the brain: relationship to calcineurin. Neuroscience62: 569–580
Tropschug M., Nicholson D., Hartl F., Kohler H., Pfanner N., Wachter E. and Neupert W. (1988) Cyclosporin A-binding protein (cyclophilin) onNeurospora crassa: one gene codes for both the cytosolic and mitochondrial forms. J. Biol. Chem.263: 14433–14444
McDonald M., Ardito T., Marks W., Kashgarian M. and Lorber M. (1992) The effect of cyclosporine administration on the cellular distribution and content of cyclophilin. Transplantation53: 460–466
Zydowsky L., Ho S., Baker C., McIntyre K. and Walsh C. (1992) Overexpression, purification, and characterization of yeast cyclophilins A and B. Protein Science1: 961–969
Price E., Zydowsky L., Jin M., Baker C., McKeon F. and Walsh C. (1991) Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc. Natl Acad. Sci. USA88: 1903–1907
Liu J. and Walsh C. (1990) Peptidyl-prolyl cis-trans-isomerase fromEscherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc. Natl Acad. Sci. USA87: 4028–4032
Hayano T., Takahashi N., Kato S., Maki N. and Suzuki M. (1991) Two distinct forms of peptidylprolyl-cis-trans-isomearse are expressed separately in periplasmic and cytoplasmic compartments ofEscherichia coli cells. Biochemistry30: 3041–3048
Kok R., Christoffels V., Vosman B. and Hellingwerf K. (1994) A gene ofAcinetobacter calcoacticus BD413 encodes a periplasmic peptidyl-prolylcis-trans isomerase of the cyclophilin sub-class that is not essential for growth. Biochim. biophys. Acta1219: 601–606
Caroni P., Rothenfluh A., McGlynn E. and Schneider C. (1991) S-cyclophilin: new member of the cyclophilin family associated with the secretory pathway. J. Biol. Chem.266: 10739–10742
Tanida I., Yanagida M., Maki N., Yagi S., Namiyama F., Kobayashi T., Hayano T., Takahashi N. and Suzuki M. (1991) Yeast cyclophilin-related gene encodes a nonessential second peptidyl-prolylcis-trans isomerase associated with the secretory pathway. Transplant Proc.23: 2856–2861
Takahashi N., Hayano T. and Suzuki M. (1989) Peptidyl-prolylcis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature337: 473–475
Sigal N., Dumont F., Durette P., Siekierka J., Peterson L., Rich D., Dunlap B., Staruch M., Melino M., Koprak S., Williams B. and Pisano J. (1991) Is cyclophilin involved in the immunosuppressive and nephrotoxic mechanism of action of cyclosporin A? J. Expl Med.173: 619–628
Haendler B., Keller P., Hiestand P., Kocher H., Wegmann G. and Movva N. (1989) Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene83: 39–46
Liu J., Farmer J., Lane W., Friedman J., Weissman I. and Schreiber S. (1991) Calcineurin is a common target of cyclophilin-cyclosporin A FKBP-FK506 complexes. Cell66: 1–9
Tropschug M., Barthelmess I. and Neupert W. (1989) Sensitivity to cyclosporin A is mediated by cyclophilin inNeurospora crassa andSaccharomyces serevisiae. Nature342: 953–955
Krummrei U., Bang R., Schmidtchen R. Brune K. and Band H. (1995) Cyclophilin-A is a zinc-dependent DNA binding protein in macrophages. FEBS Lett371: 47–51
Chatellard-Gruaz D., Saurat J.-H. and Siegenthaler G. (1994) Differential expression of cyclophilin isoforms during keratinocyte differentiation. Biochem. J.303: 863–867
Ratajczak T., Carrello A., Mark P., Warner B., Simpson R., Moritz R. and House A. (1993) The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 FKBP59). J. Biol. Chem.268: 13187–13192
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Montague, J.W., Cidlowski, J.A. Cellular catabolism in apoptosis: DNA degradation and endonuclease activation. Experientia 52, 957–962 (1996). https://doi.org/10.1007/BF01920104
Issue Date:
DOI: https://doi.org/10.1007/BF01920104