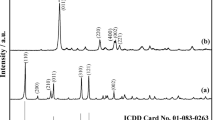
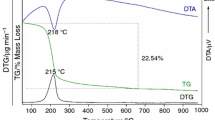
Abstract
The dehydration of CuHPO4·H2O was followed by means of thermogravimetric measurements under quasi-isothermal-quasi-isobaric conditions. The intermediates and products formed during The thermal analysis and during the calcination of the starting hydrogen phosphate in an electric furnace at various temperatures were analysed by means of thin-layer chromatography, IR spectroscopy, X-ray diffraction analysis and electron microscopy.
Zusammenfassung
Mittels thermogravimetrische Messungen wurde die Dehydration von CuHPO4·H2O unter quasiisothermen-quasiisobaren Bedingungen untersucht. Die bei der Thermoanalyse und dem Kalzinieren des ursprünglichen Hydrogenphosphates in einem elektrischen Ofen bei verschiedenen Temperaturen entstehenden Produkte und Zwischenprodukte wurde mit Hilfe von Dünnsichtchromatographie, IR-Spektroskopie, Röntgendiffraktion und Elektronenmikroskopie untersucht.
РЕжУМЕ
тЕРМОгРАВИМЕтРИЧЕс кИМ МЕтОДОМ В кВАжИИжОтЕРМИЧЕскИ х-кВАжИИжОБАРНых Усл ОВИьх БылА ИжУЧЕНА ДЕгИДРА тАцИь CuHPO4·H2O. МЕтОДАМИ тОНкОслОИНОИ хРОМАт ОгРАФИИ, Ик спЕктРОскОпИИ, РЕНтг ЕНОФАжОВОгО АНАлИжА И ЁлЕктРОННОИ МИкРОск ОпИИ ИжУЧЕНы пРОМЕжУ тОЧНыЕ И кОНЕЧНыЕ пРОДУкты, О БРАжУУЩИЕсь В пРОцЕс сЕ тЕРМИЧЕскОгО АНАлИж А, А тАкжЕ пРИ ОБжИгЕ ИсхОДНОгО гИДРОФОсФ АтА В ЁлЕктРИЧЕскОИ п ЕЧИ пРИ РАжлИЧНых тЕМпЕРАтУ РАх.
Similar content being viewed by others
References
E. Thilo and H. Grunze, Z. Anorg. Allg. Chem., 290 (1957) 209.
M. Trojan and D. Brandova, Thermochim. Acta, 88 (1985) 415.
M. Trojan and D. Brandová, Sb. VĚd, Prací, Vys. škola Chem. Technol. Pardubice, 49 (1986) 233.
M. Trojan and D. Brandová, Sb. VĚd, Prací, Vys. škola Chem. Technol. Pardubice, 47 (1985) 33.
M. Trojan, Proc. X ICPC, Bonn 1986, p. 233.
M. Trojan, Dyes and Pigments, 8 (1987) in press.
M. Trojan and P. Mazan, Czech. patent, 245 (1986) 071.
M. Trojan and D. Brandová, Chem. Listy, 81 (1987) in press.
A. Schwarzenberg, Lieb. Ann., 65 (1948) 156.
M. Basset and w. L. Badwell, J. Chem. Soc. London (1933) 854.
P. Royen and H. Brenneis, Naturwissensch., 50 (1963) 547.
H. Guerin and H. Kozicki, Bull. Soc. Chim., (1952) 782.
M. C. Ball, J. Chem. Soc., A (1968) 1113.
J. Gudennic, A. Larf, A. Riou and I. Geraull, C. R. Acad. Sci. Paris, Sect. II 299 (1984) 155.
G. Tamman, J. Prakt. Chem., 45 (1982) 469.
B. E. Robertson and C. Calvo, Acta Crystallogr., 22 (1967) 665.
B. E. Robertson and C. Calvo, Cannad. J. Chem., 46 (1968) 605.
J. GaŽo, M. Kabesová, F. Valach and M. Melnik, Koord. Chim., 2 (1976) 715.
W. L. Jolly, Preparation Inorganic Reactions, J. Wiley and Sons, New York-London-Sydney, 1965.
M. Trojan and D. Brandová, sb. VĚd. Prací, Vys. škola Chem. Technol. Pardubice, 50 (1987) in press.
J. Paulik and F. Paulik, Simultaneous Thermoanalytical Examination bv means of the Derivatograph, In Wilson-Wilson's Comprehensive Analytical Chemistry (G. Svehla, Ed.), Vol. II, Ed. W. W. Wendlandt, Elsevier, Amsterdam 1981.
F. Paulik and J. Paulik, Thermochim. Acta, 100 (1986) 23.
J. Paulik, F. Paulik and M. Arnold, Thermochim. Acta. 107 (1986) 375.
J. Paulik, F. Paulik and M. Arnold, J. Thermal Anal., 32 (1987) 301.
M. Ebert, I. Lukes and J. Nassler, Chem. Prumysl, 30/55 (1980) 402.
D. E. Corbridge and E. J. Low, J. Chem. Soc. London (1954) 493.
R. J. Melnikova, V. V. Peckovskij, E. D. Dzjuba and I. E. Malasonok, Atlas infrakrasnich spektrov fosfatov, Kondenzirovanuje fosfatu, Nauka, Moskwa 1985.
A. G. Nord and P. Kirkegaard, Chemica Scripta, 15 (1980) 27.
J. Majling, S. Ranenec and S. Durovic, Calculated powder diffraction patterns for anhydrous phosphates, Publ. “Veda”, Brtislava 1979.
M. Trojan, D. Brandová and Z. Solc, Thermochim. Acta, 85 (1985) 99.
M. Trojan, Z. Solc and M. Kuchler, Thermochim. Acta, 88 (1985) 421.
D. Brandová and M. Trojan, J. Thermal Anal., 30 (1985) 159.
J. Paulik, and F. Paulik, J. Thermal Anal., 8 (1985) 567.
Author information
Authors and Affiliations
Additional information
The authors thank Prof. E. Pungor for valuable discussions.
Rights and permissions
About this article
Cite this article
Brandová, D., Trojan, M., Arnold, M. et al. Mechanism of dehydration and condensation of CuHPO4· H2O. Journal of Thermal Analysis 34, 1449–1454 (1988). https://doi.org/10.1007/BF01914369
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01914369