Abstract
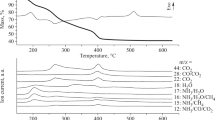
The complex K4(NH4)2 [Mo6O15(C2O4)6(H2O)4] (PAMO) was prepared and characterized on the basis of chemical analysis and IR spectral data. Its thermal decomposition was studied by using TG and DTA techniques. PAMO loses its water between 190 and 225°C followed by the decomposition of anhydrous PAMO, which takes place in three stages. The first two stages occur in the temperature ranges 225–245°C and 245–270°C, to give the intermediates with tentative compositions K12(NH4)2 [Mo18O45(CO3)4(C2O4)12 and K12[Mo18O54(CO3)2(C2O4)4] respectively, the latter then decomposing in the third stage between 270 and 335°C to give the end product, potassium trimolybadate (K2Mo3O10). The end product was characterized by chemical analysis, IR spectral and X-ray studies.
Zusammenfassung
Die Komplexverbindung K4(NH4)2[Mo6O15(C2O4)6(H2O)4] (PAMO) wurde hergestellt und auf der Basis von chemischer Analyse und IR-Spektrum characterisiert. Mittels TG und DT Techniken wurde die thermische Zersetzung untersucht. Zwischen 190 und 225°C gibt PAMO alles Wasser ab, anschlieend erfolgt in drei Schritten eine
Zersetzung des dehydratierten PAMO. Die ersten zwei Schritte verlaufen in den Temperaturbereichen 225–245°C bzw. 245–270°C und liefern Zwischenprodukte der Zusammensetzung K12(NH4)2[Mo18O45(CO3)4(C2O4)12] bzw. K12[Mo18O54(CO3)2(C2O4]. Letzteres zerfällt dann in einem dritten Schritt zwischen 270 und 335° C und liefert Kaliumtrimolybdat (K2Mo3O10) als Endprodukt, welches mittels Elementaranalyse, IR- und Röntgendiffraktionsuntersuchungen
Similar content being viewed by others
References
J. Gopalakrishnan, B. Vaswanathan and V. Srinivasan, J. Inorg. Nucl. Chem., 32 (1970) 2565.
S. P. Goel and P. N. Mehrotra, Thermochim. Acta, 70 (1983) 201.
A. I. Vogel, A Text book of Quantitative Inorganic Analysis, ELBS, 3rd edn. 1973, p. 254.
C. M. French and J. H. Garside, J. Chem. Soc., (1962) 2006.
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1970, p. 245.
J. Fujita, A. E. Martel and K. Nakamoto, J. Chem. Phys., 36 (1962) 324.
R. A. Nyquist and R. O. Kagel, Infrared Spectra of Inorganic Compounds, Academic Press, New York, London, 1971, p. 3.
C. G. Barraclough, J. Lewis and R. S. Nyholm, J. Chem. Soc., (1959) 3552.
W. P. Griffith, J. Chem. Soc., (1963) 5345.
M. Cousins and M. L. H. Green, J. Chem. Soc., (1964) 1567.
G. Sartori, C. Furlani and A. Damiani, J. Inorg. Nucl. Chem., 8 (1958) 119.
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1970, p. 70.
J. M. Reau and C. Fouassier, Bull. Soc. Chim. Fr., 2 (1971) 398.
P. Caillet, Bull. Soc. Chim. Fr., 12 (1967) 4750.
B. M. Gatehouse and P. Leverett, J. Chem. Soc. (A), (1971) 2107.
Author information
Authors and Affiliations
Additional information
The authors are thankful to Dr. M. C. Jain, Head of the Department and professor L. N. Mittal, Principal of the Institution for providing the research facilities. One of the authors (S. P. G.) is also thankful to U. G. C. for providing financial assistance.
Rights and permissions
About this article
Cite this article
Goel, S.P., Verma, G.R., Kumar, S. et al. Investigation on the formation of potassium trimolybdate via thermal decomposition of a new oxomolybdenum(VI) oxalato complex. Journal of Thermal Analysis 36, 2349–2356 (1990). https://doi.org/10.1007/BF01913632
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01913632