Abstract
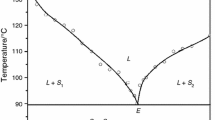
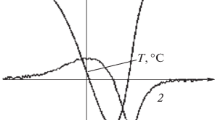
The phase diagrams of the binary systems of picric acid with naphthalene, anthracene and phenanthrene, and of α-naphthol withp-toluidine, determined by the thaw-melt method, show the formation of a molecular complex and two eutectics in each system. The heats of the pure components, eutectics and molecular complexes were determined by differential scanning calorimetry. Comparison of the experimental heats of fusion with the theoretical values calculated via the mixture law suggests cluster formation in the melts. The entropy of fusion, enthalpy of mixing and excess thermodynamic functions were also calculated from the heat of fusion data.
Zusammenfassung
Die Phasendiagramme der binären Systeme von Pikrinsäure mit Naphthalin, Anthrazen und Phenanthren sowie von α-Naphthol mit p-Aminotoluol wurden bestimmt. In jedem System zeigt sich die Bildung eines Molekülkomplexes sowie je zwei Eutektika. Mittels DSC wurden die Schmelzwärmen der reinen Komponenten, der eutektischen Mischungen und der Molekülkomplexe ermitteis. Der Vergleich der experimentell ermittelten Schmelzwärmen mit den mittels der Mischungsregel errechneten Werten läßt auf eine Clusterbildung in der Schmelze schließen. Weiterhin wurden aus den Schmelzwärmen auch Werte für Schmelzentropie und Mischungsenthalpie errechnet.
Резюме
Найденные методом ра сстаивание — расплав фазовые диаграммы дв ойных систем пикриновой кислоты с нафталином, антрацен ом и фенантреном, а такжеα-нафтола с птолуиди ном показали образовани е в каждой системе мол екулярного комплекса и двуз эвте ктик. Методом ДСК определе ны теплоты плавления чистых компонент, эвтектик и молекулярных комплексов. Сопостав ление экспериментал ьно полученных теплот пл авления с теоретичес ки вычисленными на осно ве закона смеси, предп олагает образование кластер ов в расплавах. Исходя из данных тепл от плавления были так же вычислены энтропия п лавления, энтальпия смешения и избыточны е термодинамические функции.
Similar content being viewed by others
References
N. B. Singh and K. D. Dwivedi, J. Sci. Ind. Res., 41 (1982) 96.
R. P. Rastogi, D. P. Singh, Namwar Singh and Narsingh B. Singh, Mol. Cryst. Liq. Cryst., 73 (1981) 7.
N. B. Singh and Narsingh B. Singh, Kristall und Technik, 13 (1978) 1175.
R. Elliot, Int. Met. Rev., 22 (1977) 161.
R. M. Jordan and J. D. Hunt, Met. Trans., 2 (1971) 3401.
B. Derby and J. J. Favier, Acta Met., 31 (1983) 1123.
D. J. Fisher and W. Kurz, Acta Met., 28 (1980) 777.
K. A. Jackson and J. D. Hunt, Trans. Met. Soc. AIME, 236 (1966) 1129.
R. N. Grugel and A. Hellawell, Met. Trans., 15A (1984) 1626.
W. F. Kaukler and D. O. Frazier, J. Cryst. Growth, 71 (1985) 340.
M. E. Glicksman, N. B. Singh and M. Chopra, Manuf. Space, 11 (1983) 207.
P. S. Bassi, N. K. Sharma and M. K. Sharma, Cryst. Res. Technol., 18 (1983) 1191.
P. E. Arndt, J. G. Dunn and R. L. S. Willix, Thermochim. Acta, 79 (1984) 55.
V. E. Kamper, Russian Chem. Rev., 5 (1982) 185.
U. S. Rai, O. P. Singh and Narshing B. Singh, J. Chim. Phys., 84 (1987) 483.
O. P. Singh and Narsingh B. Singh, Bull. Chem. Soc. Belg., 89 (1980) 499.
B. M. Shukla, N. P. Singh and Narsingh B. Singh, Mol. Cryst. Liq. Cryst., 104 (1984) 265.
A. Krajewska and K. Pigon, Thermochim. Acta, 41 (1980) 187.
U. S. Rai and K. D. Mandal, Acta Chimica, 125 (1988) 473.
R. P. Rastogi, N. B. Singh and K. D. Dwivedi, Ber. Bunsenges. Phys. Chem., 85 (1981) 85.
N. B. Singh, U. S. Rai and O. P. Singh, J. Cryst. Growth, 71 (1985) 353.
U. S. Rai and K. D. Mandal, Cryst. Res. Technol., 23 (1988) 871.
U. S. Rai, O. P. Singh and Narsingh B. Singh, Can. J. Chem., 65 (1987) 2639.
U. S. Rai, O. P. Singh, N. P. Singh and Narsingh B. Singh, Thermochim. Acta, 71 (1983) 373.
N. P. Singh, B. M. Shukla, Namwar Singh and Narsingh B. Singh, J. Chem. Eng. Data, 30 (1985) 49.
Namwar Singh, Narsingh B. Singh, U. S. Rai and O. P. Singh, Thermochim. Acta, 95 (1985) 291.
S. Glasstone, “Thermodynamics for Chemists”, D. Van Nostrand Company, Inc., 1960, p. 375.
Author information
Authors and Affiliations
Additional information
Thanks are due to Prof. I. S. Ahuja, Head, Chemistry Department, Banaras Hindu University, for providing research facilities. Thanks are also due to CSIR, New Delhi, for financial assistance.
Rights and permissions
About this article
Cite this article
Rai, U.S., Mandal, K.D. & Singh, N.P. Thermochemical studies on organic eutectics and molecular complexes. Journal of Thermal Analysis 35, 1687–1697 (1989). https://doi.org/10.1007/BF01912943
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01912943