Abstract
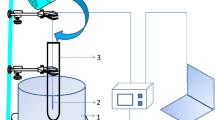
The kinetics of the thermal decomposition of the addition compound of potassium carbonate with hydrogen peroxide was carried out using a fixed bed flow reactor with gas-chromatography. The experimental results can be best represented over the whole range of the decomposition, by the equation-In (1−α)=kt, whereα=degree of decomposition,t=time (min.), andk=rate constant (min.−1). The activation energy and the pre-exponential factor for 0<α<0.8, are 74.3 kJ/mol and 7.8 · 109, and for the decay period (0.8<α<1.0), 77.3 kJ/mol and 1.38 · 1010, respectively.
Résumé
On a étudié, par chromatographie en phase gazeuse, la cinétique de la décomposition thermique du composé d'addition du carbonate de potassium avec de l'eau oxygénée, en se servant d'un réacteur à écoulement, type lit fixe.
Les résultats expérimentaux peuvent être décrits avec un bon ajustement dans tout l'intervalle de décomposition, par l'équation (I) -ln (1−α)=kt;α=degré de décomposition,t=temps (min) etk=constante de vitesse (min−1).
L'énergie d'activation et le facteur préexponentiel sont pour 0<α<0.8, respectivement égaux à 74.3 et 7.8 · 109 kJ/mole, et pour la période d'amortissement (0.8 <α<1.0) à 77.3 et 1.38 · 1010 kJ/mole.
Zusammenfassung
Die Kinetik der thermischen Zersetzung der Additionsverbindung von Kaliumcarbonat mit Wasserstoffperoxid wurde unter Anwendung eines Fixbett-Durchfluß-reaktors durch Gaschromatographie untersucht.
Die Versuchsergebnisse können im ganzen Bereich der Zersetzung am besten durch die Gleichung (I) -ln (1−α)=kt beschrieben werden;α=Zersetzungsgrad,t=Zeit (min) undk=Geschwindigkeitskonstante (min−1).
Die Aktivierungsenergie und der präexponentielle Faktor betragen, für 0<α<0.8, 74.3 kJ/Mol, bzw. 7.8 · 109 und für die Periode des Abklingens (0.8<α<1.0) 77.3 kJ/Mol, bzw. 1.38 · 1010.
Резюме
Исследована кинетик а термического разложения продукта присоединения карбо ната калия и перекиси водо рода, используя упорн ый реактор для непрерывного про цесса совместно с газовым х роматографом. Во всей области разложения эксперим ентальные результаты наилучие могут быть представл ены уравнением -ln(1−α)=кt, гдеα-степень разложения,t-время (ми н.) ик-константа скоро сти (мин.−1). Энергия актива ции и предэкспоненци альный фактор для 0<αа<0.8, соотв етственно,равны 74.3 кдж /моль и 7.8. 109, а для периода распа да (0.8<αа<1.0) —77.3 кдж/моль и 1.38. 1010.
Similar content being viewed by others
References
Gmelins Handbuch der anorg. Chemie, Verlag Chemie GmbH. Weinheim, 1969, System No. 21, p. 1364, System No. 22, p. 869, System No. 24, p. 225, System No. 25, p. 237.
T. Nagaishi, T. Kaneda, M. Matsumuto andS. Yoshinaga, J. Industrial Explosives Society, Japan, 37 (1976) 84.
H. Nakamura, S. Nakamura andI. Nakamori, ibid., 36 (1975) 27.
M.Ishibashi, Experimental Quantitative Analysis, Fuzanbo, Kyoto 1963, p. 452 (in Japanese).
C. H.Bamford and C. F. H.Tipper, Comprehensive Chemical Kinetics, Elsevier Publication Corp. 1969, vol. 2, p. 390
W. E.Garner, Chemistry of the Solid State, Butterworth Science Corp., 1955, p. 211.
Author information
Authors and Affiliations
Additional information
The authors wish to express their appreciation to Dr. D. Dollimore for his valuable comments and thank Mr. H. Nakamura, Research Fellow, Department of Environmental Engineering, Kyushu Institute of Technology, for his advise.
Rights and permissions
About this article
Cite this article
Nagaishi, T., Yoshimura, J., Matsumoto, M. et al. Thermal decomposition of the addition compound of potassium carbonate with hydrogen peroxide. Journal of Thermal Analysis 18, 501–508 (1980). https://doi.org/10.1007/BF01910231
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01910231