Summary
We investigated in the isolated rat heart the influence of the gas surrounding the globally ischemic heart on transmural inhomogeneity of energy metabolism, extracellular K+ accumulation, and change of extracellular pH. Hearts were made ischemic in 100% N2 (N2-ischemia), 100% O2 (O2-ischemia) or 100% CO2 (CO2-ischemia). We measured: 1) Midmural, subepicardial, and epicardial changes of extracellular [K+] and pH during successive 6-min periods of global ischemia, and 2) content of creatinephosphate (CrP) in consecutive tissue sections of 100 μm, from the subepicardium after 10 min of ischemia.
-
A)
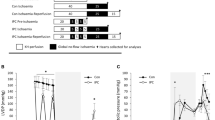
During O2-ischemia both extracellular [K+] and change of pH in the subepicardium are significantly less than in the midmyocardium. During N2-ischemia only minor differences exist in [K+] and pH between the subepicardium and the midmyocardium. During CO2-ischemia midmural and subepicardial [K+] are similar to those during N2-ischemia. The midmural change of pH resembles that during N2-ischemia; subepicardial change of pH, however, was slightly larger. Midmural changes in [K+] and pH were not influenced by the nature of the surrounding gas.
-
B)
After 10 min of O2-ischemia a gradient of tissue content of CrP extends from the epicardium (CrP about 30 μmoles/g dry weight) to a distance of about 1000 μm (CrP 1 μmoles/g dry weight). In N2-and CO2-ischemia a CrP gradient is absent; CrP is appreciably less than 1 μmoles/g dry weight at any distances from the epicardium.
-
C)
We conclude that diffusion of O2 into the myocardium and of CO2 from the myocardium affects transmural gradients of [K+], pH, and energy metabolism during ischemia. Local availability of O2 increases the capacity of the ischemic tissue to generate high energy phosphates and mitigates ischemia-induced changes of transsarcolemmal ion gradients.
Similar content being viewed by others
References
de Bakker JMT, van Capelle FJL, Janse MJ, Wilde AAM, Coronel R, Becker AE, Dingemans KP, van Hemel NM, Hauer RNW (1988) Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation 77/3:589–606
Cardinal R, Janse MJ, van Eede I, Werner G, Naumann d'Alnoncourt C, Durrer D (1981) The effects of lidocaine on intracellular and extracellular potentials, activation, and ventricular arrhythmias during acute regional ischemia in the isolated porcine heart. Circ Res 49:792–806
Coronel R, Fiolet JWT, Wilms-Schopman FJG, Schaapherder AFM, Johnson TA, Gettes LS, Janse MJ (1988) Distribution of extracellular potassium and its relation to electrophysiologic changes during acute myocardial ischemia in the isolated perfused porcine heart. Circulation 77/5:1125–1138
Estes EH, Entman ML, Dixon HB, Hackel DB, Durham NC (1966) The vascular supply of the left ventricular wall. Am Heart J 71:58–67
Fenoglio JJ, Duc Pham T, Harken AM, Horowitz LN, Josephson ME, Wit AL (1983) Recurrent sustained ventricular techycardia: structure and ultrastructure of subendocardial regions in which tachycardia originates. Circulation 68:518–533.
Fiolet JWT, Baartscheer A, Schumacher CA, Coronel R, ter Welle HF (1984) The change of the free energy of ATP hydrolysis during global ischemia and anoxia in the rat heart. Its possible role in the regulation of transsarcolemnal sodium and potassium gradients. J Mol Cell Cardiol 16:1023–1036
Fiolet JWT, Baartscheer A, Schumacher CA, ter Welle HF, Krieger WJG (1985) Transmural inhomogeneity of energy metabolism during acute global ischemia in the isolated rat heart; dependence on environmental conditions. J Mol Cell Cardiol 17:87–92
Fleet WF, Johnson TA, Graebner CA, Gettes LS (1985) Effect of serial brief ischemic episodes on extracellular K+, pH and activation in the pig. Circulation 72:922–932
Gardner PI, Ursell PC, Duc Pham T, Fenoglio JJ, Wit AL (1984) Experimental chronic ventricular tachycardia: anatomic and electrophysiologic substrates. In: Josephson ME, Wellens HJJ (eds) Tachycardias: Mechanisms, diagnosis, treatment. Lea and Febiger, Philadelphia, p 29
Gevers W (1977) Generation of protons by metabolic processes in heart cells. J Mol Cell Cardiol 9:867–874
Hill JL, Gettes LS (1980) Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation 61/4:768–778
Ichihara K, Haga N, Abiko Y (1984) Is ischemia-induced pH decrease of dog myocardium respiratory or metabolic acidosis? Am J Physiol 246:H652-H657
Kleber AG (1984) Extracellular potassium accumulation in acute myocardial ischemia. J Mol Cell Cardiol 16:389–393
Kleber AG, Riegger CB, Janse MJ (1987) Extracellular K+ and H+ shifts in early ischemia: mechanisms and relation to changes in impulse propagation. J Mol Cell Cardiol 19 S. V.:35–44
Kleber AG, Weber RJ (1985) Myocardial ischemia: dependence of resting membrane potential and extracellular K+ on pH. Experientia 41:830
Patterson RE, Kirk ES (1983) Analysis of coronary collateral structure, function and ischemic border zones in pigs. Am J Physiol 244:H23
Patterson RE, Weintraub WS, Halgash DA, Miao J, Rogers JR, Kupersmith J (1982) Spatial distribution of [C14]-Lidocaine and blood flow in transmural and lateral border zones of ischemic canine myocardium. Am J Cardiol 50:63–73
Schoemig A, Dart AM, Dietz R, Mayer E, Kuebler W (1984) Release of endogenous catecholamines in the ischemic myocardium of the rat. Circ Res 55:689–701
Steenbergen C, Delecuw G, Rich T, Williamson JR (1977) Effects of acidosis and ischemia on contractility and intracellular pH of rat heart. Circ Res 41:849–858
Weiss J, Shine KI (1982) [K+]o accumulation and electrophysiological alterations during early myocardial ischemia. Am J Physiol 243:H318-H327
Weiss J, Shine KI (1982) Extracellular K+ accumulation during myocardial ischemia in isolated rabbit heart. Am J Physiol 242:H619-H628
Whalen WJ (1969) The pO2 in isolated muscle measured with an intracellular electrode. In: Lavallee M, Schanne OF, Hebert NC (eds) Glass Microelectrodes. J Wiley & Sons Inc, New York, pp 396–403
Wiegand V, Guggi M, Meesmann W, Kessler M, Greitschus F (1979) Extracellular potassium activity changes in the canine myocardium after acute coronary occlusion and the influence of beta-blockade. Cardiovasc Res 13:297
Wilensky RL, Tranum-Jernsen J, Coronel R, Wilde AAM, Fiolet JWT, Janse MJ (1986) The subendocardial border zone during acute ischemia of the rabbit heart: an electrophysiologic, metabolic, and morphologic correlative study. Circulation 74/5:1137–1146
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schaapherder, A.F.M., Schumacher, C.A., Coronel, R. et al. Transmural inhomogeneity of extracellular [K+] and pH and myocardial energy metabolism in the isolated rat heart during acute global ischemia; dependence on gaseous environment. Basic Res Cardiol 85, 33–44 (1990). https://doi.org/10.1007/BF01907012
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01907012