Abstract
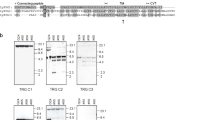
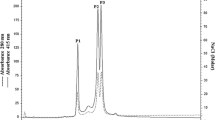
Hemoglobinβ chains were isolated from the catfishParasilurus asotus, the scadDecapterus maruadsi, the filefishThamnaconus modestus, and the scorpaenoidSebastiscus marmoratus by reverse-phase chromatography, and the N-terminal sequences were determined. To obtain the complete amino acid sequence, a 20-meric redundant consensus primer based on the N-terminal amino acid sequences of theβ chains was designed. Using this primer and oligo-dT adaptor, we amplified successfully the β-chain cDNAs of about 600 bp from the four fishes. The amplified products fromParasilurus andDecapterus were subcloned in theSmaI site of pUC18 and cDNA-derived amino acid sequences of 147 residues were determined, of which 69 and 76 residues, respectively, were identified by the chemical amino acid sequencing of internal peptides. Thus this PCR methodology using the consensus primer should be widely applicable for amplifying hemoglobinβ chains from teleosts.
Similar content being viewed by others
References
Aschauer, H., Weber, R. E., and Braunitzer, G. (1985).Biol. Chem. Hoppe-Seyler 366, 589–599.
Barra, D., Petruzzelli, R., Bossa, F., and Brunori, M. (1983).Biochim. Biophys. Acta 742, 72–77.
Chomczynski, P., and Sacchi, N. (1987).Anal. Biochem. 162, 156–159.
D'Avino, R., and Di Prisco, G. (1989).Eur. J. Biochem. 179, 699–705.
D'Avino, R., Caruso, C., Schinina, M. E., Rutigliano, B., Romano, M., Camardella, L., Bosa, F., Barra, D., and Di Prisco, G. (1989).FEBS Lett. 250, 53–56.
Felsenstein, J. (1993). PHYLIP (Phylogeny Inference Package) version 3.5c [Distributed by the author, Department of Genetics, University of Washington, Seattle, Washington].
Feng, D. F., and Doolittle, R. F. (1987).J. Mol. Evol. 25, 351–360.
Fisher, W. K., Nash, A. R., and Thompson, E. O. P. (1977).Aust. J. Biol. Sci. 30, 487–506.
Gorr, T., Kleinschmidt, T., Sgouros, J. G., and Kasang, L. (1991).Biol. Chem. Hoppe-Seyler 372, 599–612.
Grujic-Injac, B., Braunitzer, G., and Stangl, A. (1980).Hoppe-Seyler's Z. Physiol. Chem. 361, 1629–1639.
Huber, F., and Braunitzer, G. (1989a).Biol. Chem. Hoppe-Seyler 370, 245–250.
Huber, F., and Braunitzer, G. (1989b).Biol. Chem. Hoppe-Seyler 370, 831–838.
Lilijeqvist, G., Braunitzer, G., and Paleus, S. (1979).Hoppe-Seyler's Z. Physiol. Chem. 360, 125–135.
Petruzzelli, R., Barra, D., Goffredo, B. M., Bossa, F., Coletta, M., and Brunori, M. (1984).Biochim. Biophys. Acta 789, 69–73.
Rodewald, K., Stangl, A., and Braunitzer, G. (1984).Hoppe-Seyler's Z. Physiol. Chem. 365, 639–649.
Rodewald, K., Oberthur, W., and Braunitzer, G. (1987).Biol. Chem. Hoppe-Seyler 368, 795–805.
Rodewald, K., and Braunitzer, G. (1984).Hoppe-Seyler's Z. Physiol. Chem. 365, 95–104.
Saiki, R. K., Gelfand, D., Stoffel, S., Scharf, S., Higuchi, R., Horn, G., Mullis, K., and Erlich, H. (1988).Science 239, 487–491.
Suzuki, T., Furukohri, T., and Gotoh, T. (1985).J. Biol. Chem. 260, 3145–3154.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, T., Nishikawa, T. PCR amplification of cDNAs of fish hemoglobin β chains using a consensus primer: cDNA-derived amino acid sequences of β chains from the catfishParasilurus asotus and the scadDecapterus maruadsi. J Protein Chem 15, 389–394 (1996). https://doi.org/10.1007/BF01886865
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01886865