Summary
Growth of Lotus nodule bacteria (Strain LC 296) was temporarily suppressed in a yeast-extract mineral-salts medium containing 2,4-DB at 50 and 100 µg/ml. However, the 2,4-DB concentration needed to completely suppress the growth of the bacteria appeared to be above 500 µg/ml. When added during the logarithmic phase of growth 2,4-DB at 50 and 100 µg/ml did not inhibit the growth of the cells, so that the growth-inhibiting effect of the herbicide apparently was directed primarily against lag phase cells. Pre-incubation of rhizobia in a medium containing 2,4-DB at concentrations up to 10 µg/ml did not affect the capacity of the bacteria to effectively nodulate their host plant.
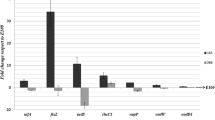
Attempts to induce the rhizobia to utilize 2,4-DB as a sole carbon source failed. Nevertheless, when theLotus nodule bacteria (LC 296) were grown in a medium containing 2,4-DB-1-C14, C14O2 was released. Similar results were obtained with other strains of the Lotus rhizobia and other species of Rhizobium. C14O2 evolution was also demonstrated when radioactive 2,4-DB was added to both intact (excised) and crushed trefoil nodules. The results indicate that the nodule bacteria are capable of degrading 2,4-DB; although the mechanism is still obscure it does not appear to be via cleavage of the ether link.
Similar content being viewed by others
References
Alexander, M., Biodegradation: Problems of molecular recalcitrance and microbial fallibility. Adv. Applied Microbiol.7, 35–80 (1965).
Alexander, M. and Aleem, M. I. H., Effect of chemical structure on microbial decomposition of aromatic herbicides. Agr. Food Chem.9, 44–47 (1961).
Audus, L. J., Biological detoxification of 2,4-dichlorophenoxyacetic acid in soil. Plant and Soil2, 31–36 (1949).
Audus, L. J., The biological detoxification of hormone herbicides in soil. Plant and Soil3, 170–192 (1951).
Burger, K., MacRae, I. C., and Alexander, M., Decomposition of phenoxyalkylcarboxylic acids. Soil Sci. Soc. Amer. Proc.26, 243–246 (1962).
Carlyle, R. E., and Thorpe, J. D., Some effects of ammonium and sodium 2,4-dichlorophenoxyacetates on legumes andRhizobium bacteria. J. Amer. Soc. Agron.39, 929–936 (1947).
Fletcher, W. W., Dickenson, P. B., and Raymond, J. C., The effect of certain hormone herbicides on the growth and nodulation ofTrifolium repens sylvestre in aseptic culture. Phyton 7, 121–130 (1956).
Fletcher, W. W., Dickenson, P. B., Forrest, J. D., and Raymond, J. C., The effect of soil-applications of certain substituted phenoxyacetic acid and phenoxybutyric acids on the growth and nodulation ofTrifolium repens sylvestre. Phyton9, 41–46 (1957).
Fletcher, W. W. and Raymond, J. C., Toxicity and breakdown of ‘hormone’ herbicides. Nature178, 151–152 (1956).
Garcia, M. M. and Jordan, D. C., The action of 2,4-DB and dalapon on the symbiotic properties ofLotus corniculatus (birdsfoot trefoil). Plant and Soil30, 317–334 (1969).
Gutenmann, W. H., Loos, M. A., Alexander, M. and Lisk, D. J., Beta oxidation of phenoxy-alkanoic acids in soil. Soil Sci. Soc. Am. Proc.28, 205–207 (1964).
Jensen, H. L., and Petersen, H. I., Decomposition of hormone herbicides by bacteria. Acta Agr. Scand.2, 214–231 (1952).
Jordan, D. C. and Coulter, W. H., On the cytology and synthetic capacities of natural and artificially produced bacteroids ofRhizobium leguminosarum. Can. J. Microbiol.11, 709–720 (1965).
MacRae, I. C. and Alexander, M., Metabolism of phenoxyalkyl carboxylic acids by aFlavobacterium species, J. Bacteriol.86, 1231–1235 (1963).
MacRae, I. C. and Alexander, M., Herbicide degradation. Microbial degradation of selected herbicides in soil. J. Agr. Food Chem.13, 72–76 (1965).
MacRae, I. C., Alexander, M. and Rovira, A. D., The decomposition of 4-(2,4-dichlorophenoxy) butyric acid byFlavobacterium sp. J. Gen. Microbiol.32, 69–76 (1963).
Newman, A. S., and Thomas, S. R., Decomposition of 2,4-dichlorophenoxyacetic acid in soil and liquid media. Soil Sci. Soc. Am. Proc.14, 161–164 (1949).
Nickell, L. G. and English, A. R., Effect of maleic hydrazide on soil bacteria and other soil organisms. Weeds2, 190–195 (1953).
Nilsson, P. E., The influence of antibiotics and antagonists on symbiotic nitrogen fixation of legume cultures. Kungl. Lantbrukshogkol. Ann.23, 219–253 (1957).
Umbreit, W. W., Burris, R. H., and Stauffer, J. F., Manometric Techniques. 3rd ed., Burgess Publishing Co., Minneapolis (1957).
Webley, D. M. Duff, R. B., and Farmer, V. C., Beta oxidation of fatty acids byNocardia opaca. J. Gen. Microbiol.13, 361–369 (1955).
Webley, D. M., Duff, R. B., and Farmer, V. C., Formation of a β-hydroxy acid on an intermediate in the microbiological conversion of monochlorophenoxybutyric acids to the corresponding acetic acids. Nature179, 1130–1131 (1957).
Webley, D. M., Duff, R. B., and Farmer, V. C., The influence of chemical structure on β-oxidation by soil Nocardias. J. Gen. Microbiol.18, 733–746 (1958).
Whiteside, J. S., and Alexander, M., Measurement of microbiological effects of herbicides. Weeds8, 204–213 (1960).
Wilson, P. W., Respiratory enzyme systems in symbiotic fixation. I. The ‘resting cell’ technique as a method for study of bacterial metabolism. J. Bacteriol.35, 601–623 (1938).
Wroebel, T., The influence of 2,4-D onRhizobium. Acta Microbiol. Polon.1, 39–41 (1952).
Author information
Authors and Affiliations
Additional information
4-(2,4-dichlorophenoxy) butyric acid.
Rights and permissions
About this article
Cite this article
Jordan, D.C., Garcia, M.M. Interactions between 2,4-DB * and the root-nodule bacteria ofLotus corniculatus . Plant Soil 30, 360–372 (1969). https://doi.org/10.1007/BF01881963
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01881963