Abstract
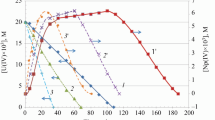
Treatment of a sulfuric acid solution of tetravalent uranium. containing 0.8 N H2SO4, in the absence of O2 and at a concentration of U+4 ∼100 mg-equiv/liter, with Co60 γ-radiation gave an oxidation yield of U+4 close to 5.0. U+4 was not oxidized by the molecular ion H +2 even with greatly increased acidity of the solution and a decreased U+4 concentration. A decrease in U+4 concentration resulted in a lower yield mainly due to recombination of the H and OH radicals. An equation for the relation of G to [U+4] is put forward which agrees with experimental data, and a ratio involving the rate constants of the three reactions H + OH, H + H and U+4 + OH was found. A decrease in the yield G was observed on increasing the U+4 concentration above 110 mg-equiv/liter. This phenomenon is apparently explained by the reduction of U+4 radicals by H. We considered, some mechanism by which uranyl ions may retard U+4 oxidation. As a result of this consideration we were able to calculate the ratio of the rate constants of the reactions UO +22 + H and H + H and a ratio involving the rate constants of the reactions UO +22 + OH, H + OH and H + H.
An equation based on the mechanism suggested for the oxidation of tetravalent uranium in dilute solution in the presence of uranyl ion has been deduced for the relation of G to [U+4] and [UO +22 ] and this agrees with the experimental data for a wide range of concentrations.
Similar content being viewed by others
Literature cited
J. W. Boyle, W. F. Kieffer, C. J. Hochanadel, T. J. Sworski, J. A. Ghormley. Proc. Intern. Conf. Peaceful Uses Atomic Energy, Geneva, 1955, U. N. (New York, 1956) v. 7, p. 576.
M. Haissinsky, J. chim. phys. et phys.-chim. biol. 53, 542 (1956).
M. Haissinsky, M. Duflo, J. chim. phys. et phys.-chim. biol. 53, 970 (1956).
A. O. Allen, Radiation Res. I, 85 (1954).
V. G. Firsov, J. Atomic Energy 2, 182 (1957).
H. G. Heal, Nature 157, 225 (1946).
H. G. Heal, G. N. Thomas, Trans. Faraday Soc. 45, 11 (1949).
T. Rigg, G. Stein, J. Weiss, Proc. Roy. Soc. A211, 375 (1952).
T. Rigg, G. Stein, J. Weiss, J. Amer. Chem. Soc. 77, No. 17, 4526 (1955).
Rights and permissions
About this article
Cite this article
Firsov, V.G., Ershler, B.V. Radiation oxidation of tetravalent uranium solutions. The Soviet Journal of Atomic Energy 4, 451–457 (1958). https://doi.org/10.1007/BF01880568
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01880568