Abstract
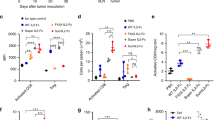
The administration of mTNFα and hIL-1α was investigated for their potential to increase the anti-tumor activity of AMN-anti-Ly-2.1 against the Ly-2.1+ murine thymoma ITT(1) 75 NS E3. Dose response studies using mTNFα alone demonstrated a single 10μg iv injection produced 30% inhibition in tumor size while 3 doses of 1μg administered on alternative days produced 70% tumor inhibition. By contrast, hIL-1α was unable to significantly reduce E3 tumor size using single doses up to 10μg or a total of 30μg administered in 3 doses (iv or ip). However, intratumor injection of hIL-1α (20μg injected in 2 doses) produced 20% inhibition in tumor size. Combination therapy using AMN immunoconjugates with mTNFα showed enhanced antitumor activity compared to each agent alone. Biodistribution studies revealed that anti-tumor activity, was due to increased localization (2–3 fold) of AMN immunoconjugates in the presence of mTNF-α whereas huIL-1α was without effect unless accompanying toxicity was seen. Clearly for this tumor, mTNFα potentiated the effects of AMN immunoconjugates. Despite the shared biological properties of these cytokines, mTNFα is superior to hIL-α for augmenting drug immunoconjugate.
Similar content being viewed by others
Abbreviations
- AMN:
-
Aminopterin
- CBF1 :
-
(C57BL6xBALB/c)F1 mice
- DMSO:
-
Dimethyl sulfoxide
- E3:
-
ITT(1)75NS E3
- HAMA:
-
human-anti-mouse antibody
- i.p.:
-
intraperitoneal(ly)
- I.T.:
-
intratumor
- i.v.:
-
intravenous(ly)
- hIL-1α :
-
recombinant human Interleukin-1-alpha
- MoAb:
-
monoclonal antibody
- PBS:
-
phosphate buffered saline
- SE:
-
standard error
- s.c.:
-
subcutaneous(ly)
- mTNF:
-
recombinant murine tumor necrosis factor-alpha
References
Double JA and Bibby MC. Therapeutic index: A vital component in selection of anti-cancer agents for clinical trial. J Natl Cancer Inst 1989; 81: 988–94.
Pietersz GA, Krauer KG, Toohey B, Smyth MJ and McKenzie IFC. Biodistribution of N-Acetylmelphalan-monoclonal antibody conjugates in mice. Antibody Immuoconj Radiopharm 1990; 3(1): 27–35.
Pietersz GA, Smyth MJ, Kanellos J, Cunningham Z, Sacks NPM. and McKenzie IFC. Preclinical and clinical studies with a variety of immunoconjugates. Antibody Immunoconj Radiopharm 1988; 1: 79–103.
Goerlach A, Krauer KG, McKenzie IFC and Pietersz GA.In vitro antitumor activity of 2′-Deoxy-fluorouridine-monoclonal antibody conjugates. Bioconj Chem 1991; 2: 96–101.
Rowland GF, Simmonds PG, Gore VA, Moresden CH and Smith WD. Drug localization and growth inhibition of vindesine-monoclonal anti-CEA conjugates in a human tumour xenograft. Cancer Immunol Immunother 1985; 21: 183–7.
Tjandra JJ, Pietersz GA, Teh JG, Cuthbertson AM, Sullivan TR, Penfold C and McKenzie IFC. Phase I clinical trial of drug-monoclonal antibody conjugates in patients with advanced colorectal carcinoma — a preliminary report. Surgery 1989; 106: 533–45.
Elias DJ, Hirschowitz L, Kline LE, Kroener JF, Dillman RO, Walker LE, Robb JA and Timm RM. Phase I clinical comparative study of monoclonal antibody KS1/4 and KS1/4-methotrexate immunoconjugate in patients with non-small lung carcinoma. Cancer Res 1990; 50: 4154–9.
Smyth MJ, Pietersz GA and McKenzie IFC. Use of vasoactive agents to increase tumor perfusion and the anti-tumor efficacy of drug-monoclonal antibody conjugates. J Natl Cancer Inst 1987; 79: 1367–73.
Russell SM, Krauer KG, McKenzie IFC and Pietersz GA. Effect of tumor necrosis factor on the anti-tumor efficacy and toxicity of aminopterin — monoclonal antibody conjugates: Parameters for optimization of therapy. Cancer Res 1990; 50: 6028–33.
Schiller JH, Storer ES, Witt PL, Alberti P, Tombes MB, Arzoomanian R, Procto RA, McCarthy D, Brown RR, Voss SD, Remick SC, Grem JL, Borden EC and Trump DL. Biological and clinical effects of intravenous tumor necrosis factor-α administered three times weekly. Cancer Res 1991; 51: 1651–8.
Bukowski RM, Sergi JS, Sharfman WJ, Budd GT, Murthy S, Barna B, Medendorp SV, Yen-Lieberman B, Gibson V, Bauer L and Valenzuela R. Phase I trial of natural human interferonβ in metastatic malignancy. Cancer Res 1991; 51: 836–40.
Sugarman BJ, Aggarwal BB, Hase FE, Figari IS, Palladino MA and Shephard HM. Recombinant human tumor necrosis factor-alpha affects on proliferation of normal and transformed cellsin vitro. Science 1985; 230: 943–5.
Watanabe N, Nitsu Y, Umeno H et al. Toxic effects of tumor necrosis factor on tumor vasculature in mice. Cancer Res 1988; 48: 2179–83.
Semenzato G. Tumor necrosis factor: a cytokine with multiple biological activities. Br J Cancer 1990; 61: 354–61.
Dinarello CA. Interleukin-1. Rev Infect Dis 1984; 6: 51–95.
Dinarello CA, Cannon JG, Mier JU, Bernheim HA, LoPreste G, Lynn DL, Love RN, Webb AC, Auron PE, Reuben RC, Rich A, Wolff SM and Putney SD. Multiple biological activities of human recombinant Interleukin-1. J Clin Invest 1986; 77: 1734–39.
Morrissey P, Charrier K, Bressler L and Alpert A. The influence of hIL-1α treatment on the reconstitution of the hemopoietic and immune systems after sublethal radiation. J Immunol 1988; 140: 4204–10.
Eppstein DA, Kurahara CG, Bruno NA and Terell TG. Prevention of doxorubicin-induced hematotoxicity in mice by interleukin-1. Cancer Res 1989; 49: 3955–60.
Martin S, Maruta K, Burkart V, Gillis S and Kolb H. hIL-1α: and IFN-γ increase vascular permeability. Immunol 1988; 64: 301–5.
Onosaki K, Matsushima K, Aggarwal BB and Oppenheim JJ. Human interleukin-1 is a cytocidal factor several tumor cell-lines. J Immunol 1985; 135: 3962–68.
Nakamura S, Nakata K, Kashimoto S, Yoshida H and Yamada M. Antitumor effect of recombinant human interleukin-1 alpha against murine syngeneic tumors. Jpn J Cancer Res 1986; 77: 767–773.
Nakamura S, Kashimoto S, Kajikawa F and Nakata K. Combination effect of recombinant human interleukin-1α with antitumor drugs on syngeneic tumors in mice. Cancer Res 1991: 51; 215–21.
Kilian PL, Kaffka KL, Stern AS, Woehle D, Benjamin WR, Dechiara TM, Gubler U, Farrar JJ, Mizel SB and Lomedico PT. Interleukin-1α and Interleukin-1β bind to the same receptor on T cells. J Immunol 1986; 136: 4509–14.
Hogarth PM, Henning MM and McKenzie IFC. Alloantigenic phenotype of radiation induced thymomas in the mouse. J Natl Cancer Inst 1982; 69: 619–25.
Kanellos J, Pietersz GA, Cunningham Z and McKenzie IFC. Antitumor activity of aminopterin monoclonal antibody conjugates:in vivo andin vitro comparison with methotrexate monoclonal antibody conjugates. Immunol Cell Biol 1987; 65: 483–93.
Hogarth PM, Edwards J, McKenzie IFC, Goding JW and Liew FY. Monoclonal antibodies to murine Ly-2.1 cell-surface antigen. Immunol 1982; 46: 135–44.
Hunter WM and Greenwood FC. Preparation of Iodine 131-labelled human growth hormone of high specificity activity. Nature 1962; 194: 495–8.
Aicher KP, et al. Contrast-enhanced magnetic resonance imaging of tumour bearing mice treated with human recombinant tumour necrosis factorα. Cancer Res 1990; 50: 7376–81.
Shimomura K, Manda T, Mukomoto S, Kaboyashi K, Nakano K and Mori J. Recombinant human tumour necrosis factor-α; Thrombus formation is a cause of anti-tumour activity. Int J Cancer 1988; 41: 243–7.
Watanabe N, Niitsu Y, Umeno H et al. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res 1988; 48: 2179–83.
Braunschweiger PG, Johnson CS, Kumar N, Ord V and Furmanski P. Anti-tumor effects of recombinant human interleukin-1α in RIF-1 and Panc 02 solid tumors. Cancer Res 1988; 48: 6011–6.
Nakata K, Kashimoto S, Yoshida H, Oku T and Nakamura S. Augmented antitumor effect of recombinant human interleukin-1α by indomethacin. Cancer Res 1988; 48: 584–8.
Miyamoto T and Wu S. Antitumor activity of recombinant human interleukin-1 against heterotransplanted human non-hodgkin lymphomas in nude mice. Jpn J Cancer Res 1990; 81: 1175–83.
Hakkert BC, Kuijpers TW, Leeuwenberg JFM, Van Mourik JA and Roos D. Neutrophil and monocyte adherence to and migration across monolayers of cytkine activated endothelial cells: the contribution of CD18, ELAM-1 and VLA-4. Blood 1991; 2721–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krauer, K.G., Pietersz, G.A. The comparative effects of IL-1 and TNF on AMN-anti-Ly 2.1 immunoconjugate therapy. Biotherapy 6, 139–147 (1993). https://doi.org/10.1007/BF01877427
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01877427