Abstract
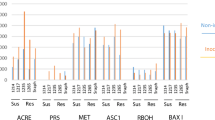
Onset of acquired resistance of barley (Hordeum vulgare) chemically induced by 2,6-dichloroisonicotinic acid (DCINA) correlated with the accumulation of mRNA homologous to cDNA pHvJ256 which codes for a soluble leaf-thionin with a Mr. of 6 kDa [Wasternacket al., 1994a]. In the present work, we extend this finding by showing that the thionin transcript also accumulated following treatment of barley with the resistance-inducing compounds 3,5-dichlorosalicylic acid (DCSA), salicylic acid (SA), and an extract fromBacillus subtilis. The polypeptide showed antifungal activity against the biotrophic cereal pathogensErysiphe graminis f.sp.hordei andPuccinia graminis f.sp.tritici which may indicate a possible role in the mechanism of acquired resistance in barley. A thionin transcript hybridizing to pHvJ256 accumulated also in response to application of jasmonates, or treatments that elevated endogenous amounts of the plant growth substance, pointing to the possibility that signaling mediating defense responses in barley involves jasmonates. However, a topical spray application of jasmonic acid (JA) or jasmonate methyl ester (JM) did not protect barley leaves against infection byE. graminis. Performing a kinetic analysis by an enzyme immunoassay specific for (−)-JA, (−)-JM, and its amino acid conjugates, accumulation of jasmonates was detected in osmotically stressed barley but not at the onset of chemically induced or genetically based resistance governed by the powdery mildew resistance genesMlg, Mla 12, ormlo 5. Furthermore, the jasmonate-inducible proteins JIP-23 and JIP-60 were strongly induced following JM- but not DCINA-treatment or inoculation withE. graminis. Hence, in barley, no indications were found in favour for the previously proposed model of a lipid-based signaling pathway via jasmonates mediating expression of resistance in plants against pathogens.
Similar content being viewed by others
Abbreviations
- Egh :
-
Erysiphe graminis f.sp.hordei
- ESH:
-
elongated secondary hyphae
- DCINA:
-
2,6-dichloroisonicotinic acid
- DCSA:
-
3,5-dichlorosalicylic acid
- HR:
-
hypersensitive response
- INA:
-
isonicotinic acid
- JA:
-
jasmonic acid
- JIP:
-
jasmonate-induced protein
- JM:
-
jasmonate methyl ester
- Pgt :
-
Puccinia graminis f.sp.tritici
- PR:
-
pathogenesis related
- SA:
-
salicylic acid
References
Aist JR, Gold RE, Bayles CJ, Morrison GH, Chandra S and Israel HW (1988) Evidence that molecular components of papillae may be involved inml-o resistance to barley powdery mildew. Physiol Mol Plant Pathol 33: 17–32
Andresen I, Becker W, Schlüter K, Burges J, Parthier B and Apel K (1992) The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (Hordeum vulgare). Plant Mol Biol 19: 193–204
Becker W and Apel K (1992) Isolation and characterization of a cDNA clone encoding a novel jasmonate-induced protein of barley (Hordeum vulgare L.). Plant Mol Biol 19: 1065–1067
Bohl S and Apel K (1993) A novel fingerprint method for analysing the expression of complex multigene families of very low transcript abundance. Plant Journal 3: 887–893
Bohlmann H, Clausen S, Behnke S, Giese H, Hiller C, Reimann-Philipp U, Schrader G, Vibeke B and Apel K (1988) Leaf-specific thionins of barley — a novel class of cell wall proteins toxic to plant-pathogenic fungi and possibly involved in the defence mechanism of plants. EMBO J 7: 1559–1565
Bohlmann H and Apel K (1991) Thionins. Annu Rev Plant Physiol 42: 227–240
Boyd LA, Smith PH, Green RM and Grown JKM (1994) The relationship between the expression of defense-related genes and mildew development in barley. Mol Plant-Microbe Interactions 7: 401–410
Bunge S, Wolter J and Apel K (1992) A comparison of leaf thionin sequences of barley cultivars and wild barley species. Mol Gen Gen 231: 460–468
Chaudhry B, Müller-Uri F, Cameron-Mills V, Gough S, Simpson D, Skriver K and Mundy J (1994) The barley 60 kD jasmonate-induced protein (JIP-60) is a novel ribosome inactivating protein. Plant Journal, in press
Chester KS (1933) The problem of acquired physiological immunity in plants. Quarterly Reviews of Biology 8: 129–154
Choi D, Bostock RM, Avdishko S and Hildebrand DF (1994) Lipid-derived signals that discriminate wound- and pathogen-responsive isoprenoid pathways in plants: Methyl jasmonate and the fungal elicitor arachidonic acid induce different 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids inSolanum tuberosum (L.). Proc Natl Acad Sci USA 91: 2329–2333
Croft KPC, Jüttner F and Slusarenko AJ (1993) Volatile products of the lipoxygnease pathway evolved fromPhaseolus vulgaris (L.) leaves inoculated withPseudomonas syringae pv.phaseolicola. Plant Physiol 101: 13–24
Dittrich H, Kutchan TM and Zenk MH (1992) The jasmonate precursor 12-oxo-phytodienoic acid induces phytoalexin synthesis inPetroselinum crispum cell cultures. FEBS-Letters 309: 33–36
Ebrahim-Nesbat F, Bohl S, Heitefuss R and Apel K (1993) Thionins in cell walls and papillae of barley in compatible and incompatible interactions withErysiphe graminis f.sp.hordei. Physiol Mol Plant Pathol 43: 343–352
Ebrahim-Nesbat F, Bohl S, Kleinhofs A and Apel K (1989) Cultivar-related differences in the distribution of cell-wall-bound thionins in compatible and incompatible interactions between barley and powdery mildew. Planta 179: 203–210
Ellingboe AH (1972) Genetics and physiology of primary infection byErysiphe graminis. Phytopathology 62: 401–406
Farmer EE and Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129–134
Fernandez de Caleya R, Gonzalez B, Garcia-Olmedo F and Carbonero P (1972) Susceptibility of phytopathogenic bacteria to wheat purothioninsin vitro. Appl Microbiol 23: 988–1000
Feussner I, Ziegler J, Miersch O and Wasternack C (1994) Jasmonate- and stress-induced lipoxygenase forms in barley leaf segments (Hordeum vulgare cv. Salome). In: Proceeding of the 11th international meeting on plant lipids, Paris
Forde BG, Kreis M, Bahramian MB, Matthews JA and Mitlin BJ (1991) Molecular cloning and analysis of cDNA sequences derived from polyA+RNA from barley endosperm: identification of B. hordein related clones. Nucl Acids Res 9: 6689–6707
Freialdenhoven A, Scherag B, Hollricher K, Collinge DB, Thordal-Christensen H and Schulze-Lefert P (1994)Nar-1 andNar-2, two loci required forMla 12-specified race-specific resistance to powdery mildew in barley. Plant Cell 6: 983–994
Görg R, Hollericher K and Schulze-Lefert P (1993) Functional analysis and RFLP-mediated mapping of theMlg resistance locus in barley. Plant Journal 3: 857–866
Gundlach H, Müller MJ, Kutchan TM and Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA 89: 2389–2393
Hahn W, Jüngling S and Knogge W (1993) Cultivar-specific elicitation of barley defense reactions by the phytotoxic peptide NIP1 fromRhynchosporium secalis. Mol Plant-Microbe Interactions 6: 745–754
Hause B, zur Nieden U, Lehmann J, Wasternack C and Parthier B (1994) Intracellular localization of jasmonate-induced proteins in barley leaves. Bot Acta 107: 333–341
Hildmann T, Ebneth M, Peña-Cortés J, Sánchez-Serrano JJ and Willmitzer L (1992) General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell 4: 1157–1170
Kessmann H, Staub T, Hofman C, Maetzke T, Herzog J, Ward E, Uknes S and Ryals J (1994) Induction of systemic acquired disease resistance in plants by chemicals. Annu Rev Phytopathol 32: 439–459
Knöfel HD, Brückner C, Kramell R, Sembdner G and Schreiber K (1990) Radioimmunoassay for the natural plant growth regulator (−)-jasmonic acid. Biochem Physiol Pflanzen 186: 387–394
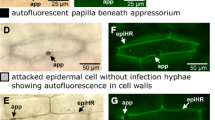
Koga H, Bushnell WR and Zeyen RJ (1990) Specificity of cell type and timing of events associated with papilla formation and the hypersensitive reaction in leaves ofHordeum vulgare attacked byErysiphe graminis f.sp.hordei. Can J Bot 68: 2344–2352
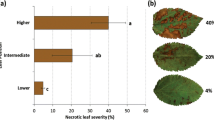
Kogel KH, Beckhove U, Dreschers J, Münch S and Rommé Y (1994) Acquired resistance in barley; The resistance mechanism induced by 2,6 dichloroisonicotinic acid is a phenocopy of a genetically based mechanism governing race-specific powdery mildew resistance. Plant Physiol 106: 1269–1277
Kølster P, Munk L, Stølen O and Løhde J (1986) Near-isogenic barley lines with genes for resistance to powdery mildew. Crop Sci 26: 903–907
Lehmann J, Atzorn R, Brückner C, Reinbothe S, Leopold J, Wasternack C and Parthier B (1995). Specific expression of endogenous jasmonate, jasmonate- and ABA-inducible transcripts and proteins in osmotically stressed barley leaf segments. Planta, in press
Maniatis T, Fritsch EF and Sambrook J (1982) Molecular Cloning: A Laboratory Manual. Cold Spring Harbour Press, New York
Métraux JP, Ahl-Goy P, Staub T, Speich T, Steinemann A, Ryals J and Ward E (1991) Induced resistance in cucumber in response to 2,6-dichloroisonicotinic acid and pathogens. In: Henecke H. and Verma DPS. (eds) Advances in Molecular Genetics of Plant-Microbe Interactions, Vol. 1 (pp 432–439) Kluwer, Dordrecht, The Netherlands
Métraux JP, Kessmann H, Ahlgoy P, Gutrella M, Ward E and Ryals J (1993) The molecular biology of systemic acquired resistance. In: Fritig B and Legrand M (eds) Mechanisms of Plant Defense Responses (pp 422–432) Kluwer Academic Publishers, Dordrecht
Müller MJ, Brodschelm W, Spannagl E and Zenk MH (1993) Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci USA 90: 7490–7494
Müller-Uri F, Parthier B and Nover L (1988) Jasmonate-induced alteration of gene expression in barley leaf segments analysed byin vivo andin vitro protein synthesis. Planta 176: 241–248
Peña-Cortés H, Willmitzer L and Sánchez-Serrano JJ (1991) Abscisic acid mediates wound induction but not developmental-specific expression of the proteinase inhibitor II gene family. Plant Cell 3: 936–972
Peña-Cortés H, Albrecht H, Prat S, Weiler E and Willmitzer L (1993) Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191: 123–128
Rebmann G, Hertig C, Bull J, Mauch F and Dudler R (1991) Cloning and sequencing of cDNAs encoding a pathogen-induced putative peroxidase of wheat (Triticum aestivum L.). Plant Mol Biol 16: 329–331
Reinbothe S, Mollenbauer B and Reinbothe C (1994a) JIPs and RIPs: The regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell 6: 1197–1209
Reinbothe S, Reinbothe C, Lehmann J, Becker W, Apel K and Parthier B (1994b) JIP-60, a methyl jasmonate-induced ribosome-inactivating protein involved in plant stress reactions. Proc Natl Acad Sci USA 91: 7012–7016
Reinbothe S, Reinbothe C, Lehmann J and Parthier B (1992) Differential accumulation of methyl jasmonate-induced mRNAs in response to abscisic acid and desiccation in barley (Hordeum vulgare). Physiol Plant 86: 49–56
Reimann-Philipp U, Schrader G, Martinoia E, Barkholt V and Apel K (1989) Intracellular thionins of barley: A second group of leaf thionins closely related to but distinct from cell wall-bound thionins. J Biol Chem 264: 8978–8984
Sahashi N and Shishiyama J (1986) Increased papilla formation, a major factor of induced resistance in the barley —Erysiphe graminis f.sp.hordei system. Can J Bot 64: 2178–2181
Schweizer P, Gees R and Mösinger E (1993) Effect of jasmonic acid on the interaction of barley (Hordeum vulgare L.) with the powdery mildewErysiphe graminis f.sp.hordei. Plant Physiol 102: 503–511
Sogaard B and JørgensenJH (1988) Genes for reaction toErysiphe graminis hordei (powdery mildew). List of barley genetic stockes. Barley Genet Newsletter 17: 120–134
Steiner U (1989) Zum Einfluß induzierter Resistenz auf den Wirt-Parasit Komplex Gerste-Echter Mehltau: Sortenabhängige Resistenzreaktion und Befalls-Verlust Relationen. Thesis, Universität Hannover
Uknes SJ, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E and Ryals J (1992) Acquired resistance inArabidopsis. Plant Cell 4: 645–656
Ward ER, Uknes, SJ, William SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Métraux JA and Ryals JA (1991) Coordinate gene activation in response to agents that induce systemic acquired resistance. Plant Cell 3: 1085–1094
Wasternack C, AtzornR, Jarosch B and KogelKH (1994a) Induction of a thionin, the jasmonate-induced 6 kDa protein of barley by 2,6-dichloroisonicotinic acid. J Phytopathology 140: 280–284
Wasternack C, Atzorn R, Blume B, Leopold J and Parthier B (1994b) Ursolic acid inhibits synthesis of jasmonate-induced proteins in barley leaves. Phytochem 35: 49–54
Weidhase RA, Kramell H, Lehmann J, Liebisch HW, Lerbs W and Parthier B (1987) Methyljasmonate-induced changes in the polypeptide pattern of senescing barley leaf segments. Plant Science 51: 177–186
Weiler EW (1986) Plant hormone immunoassay on mono-clonal and polyclonal antibodies. In: Linskens HF and Jackson JF (eds) Modern Methods of Plant Analysis: Immunology in Plant Science, Vol 4 (pp 1–17) Springer Verlag, Berlin, Heidelberg, New York
Wiberg A (1974) Genetical studies of spontaneous sources of resistance to powdery mildew in barley. Hereditas 77: 89–148
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kogel, KH., Ortel, B., Jarosch, B. et al. Resistance in barley against the powdery mildew fungus (Erysiphe graminis f.sp.hordei) is not associated with enhanced levels of endogenous jasmonates. Eur J Plant Pathol 101, 319–332 (1995). https://doi.org/10.1007/BF01874788
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01874788