Summary
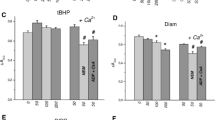
The sulfhydryl (SH) oxidant diamide activated in a concentration-dependent manner ouabain-resistant (OR), Cl-dependent K flux in both low potassium (LK) and high potassium (HK) sheep red cells as determined from the rate of zero-trans K efflux into media with Cl or Cl replaced by NO3 or methane sulfonate (CH3SO3). Diamide did not alter the OR Na efflux into choline Cl. The diamide effect on K efflux appeared after 80% of cellular glutathione (GSH) was oxidized to GSSG, its disulfide. The stimulation of K efflux was completely reversed during metabolic restitution of GSH, a process that depended on the length of exposure to and the concentration of diamide. The action of diamide on both the K∶Cl transporter and GSH was also fully reversed by the reducing agent dithiothreitol (DTT). Diamide apparently oxidized the same SH groups alkylated by N-ethylmaleimide (NEM) (Lauf, P.K. 1983.J. Membrane Biol..73:237–246). Like NEM, diamide activated K∶Cl transport several-fold more in LK cells than in HK cells, and the effect on LK cells was partially inhibited by anti-L1, the allo-antibody known to inhibit OR K fluxes.
Similar content being viewed by others
References
Bauer, J., Lauf, P.K. 1981. Thiol-dependent passive K/Cl transport in sheep red cells: III. Differential reactivity of membrane SH groups with N-ethylmaleimide and iodoacetamide.J. Membrane Biol. 73:257–261
Bergmann, W.L., Dressler, V., Haest, C.W.M., Deuticke, B. 1984. Cross-linking of SH-groups in the erythrocyte membrane enhances transbilayer reorientation of phospholipids: Evidence for a limited access of phospholipids to the reorientation sites.Biochim. Biophys. Acta 769:390–398
Beutler, E. 1971. Red Cell Metabolism. pp. 103–105. Grune & Stratton. New York-London
Deuticke, B. 1987. The role of membrane sulfhydryls in passive, mediated transport processes and for the barrier function of the erythrocyte membrane.Membrane Biochem. 6:309–316
Deuticke, B., Poser, B., Lütkemeier, P., Haest, C.W.M. 1983. Formation of aqueous pores in the human erythrocyte membrane after oxidative cross-linking of spectrin by diamide.Biochim. Biophys. Acta 731:196–210
Dressler, V., Haest, C.W.M., Plasa, G., Deuticke, B., Erusalimsky, J.D. 1984. Stabilizing factors of phospholipid asymmetry in the erythrocyte membrane.Biochim. Biophys. Acta 775:189–196
Dunham, P.B., Ellory, J.C. 1981. Passive potassium transport in low potassium sheep red cells: Dependence upon cell volume and chloride.J. Physiol (London) 318:511–530
Dunham, P.B., Stewart, G.W., Ellory, J.C. 1980. Chlorideactivated passive potassium transport in human erythrocytes.Proc. Natl. Acad. Sci. USA 77:1711–1715
Ellory, J.C., Dunham, P.B. 1980. Volume-dependent passive potassium transport in LK sheep red cells.In: Membrane Transport in Erythrocytes. Alfred Benzon Symposium, 14. U.V. Lassen, H.H. Ussing, and J.O. Wieth, editors. pp. 409–423, Munksgaard, Copenhagen
Fischer, T.M., Haest, C.W.M., Stohr, M., Kamp, D., Deuticke, B. 1978. Selective alteration of erythrocyte deformability by SH reagents.Biochim. Biophys. Acta 510: 270–282
Fisher, T.J., Tucker, E.M., Young, J.D. 1986. Relationship between cell age, glutathione and cation concentration in sheep erythrocytes with a normal and a defective transport system for amino acids.Biochim. Biophys. Acta 884:211–214
Franck, P.F.H., Opden Kamp, J.A.F., Roelofsen, B., Deenen, L.L.M. van 1986. Does diamide treatment of intact human erythrocytes cause a loss of phospholipid asymmetry?Biochim. Biophys. Acta 857:127–130
Fujise, H., Lauf, P.K. 1987. Swelling, NEM, and A23187 activate Cl−-dependent K+ transport in high-K sheep red cells.Am. J. Physiol. 252:C195-C204
Garcia-Sancho, J., Sanchez, A., Herreros, B. 1979. Stimulation of monovalent cation fluxes by electron donors in the human red cell membrane.Biochim. Biophys. Acta 556:118–130
Haest, C.W.M., Plasa, G., Kamp, D., Deuticke, B. 1978. Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane.Biochim. Biophys. Acta 509:21–32
Jung, D.W., Brierly, G.P. 1982. The redox state of pyridine nucleotides controls permeability of uncoupled mitochondria to K+ Biochem. Biophys. Res. Commun. 106:1372–1377
Kaji, D. 1986. Volume-sensitive K transport in human erythrocytes.J. Gen. Physiol. 88:719–738
Kaji, D., Kahn, T. 1985. Kinetics of Cl-dependent K influx in human erythrocytes with and without external Na: Effect of NEM.Am. J. Physiol. 249:C490-C496
Kosower, N.S., Kosower, E.M., Wertheim, B. 1969. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide.Biochem. Biophys. Res. Commun. 37:593–596
Kosower, N.S., Zipser, Y., Faltin, Z. 1982. Membrane thioldisulfide status in glucose-6-phosphate dehydrogenase deficient red cells.Biochim. Biophys. Acta 691:345–352
Kramhoft, B., Lambert, I.H., Hoffmann, E.K., Jorgensen, F. 1986. Activation of Cl-dependent K transport in Ehrlich ascites tumor cells.Am. J. Physiol. 251:C369-C379
Lauf, P.K. 1983. Thiol-dependent passive K/Cl transport in sheep red cells: I. Dependence on chloride and external K+[Rb+] ions.J. Membrane Biol. 73:237–246
Lauf, P.K. 1983. Thiol-dependent passive K/Cl transport in sheep red blood cells: V. Dependence on metabolism.Am. J. Physiol. 245:C445-C448
Lauf, P.K. 1984. Thiol-dependent passive K+Cl− transport in sheep red blood cells: VI. Functional heterogeneity and immunologic identity with volume-stimulated K+(Rb+) fluxes.J. Membrane Biol. 82:167–178
Lauf, P.K. 1985. On the relationship between volume- and thiol-stimulated K+Cl− fluxes in red cell membranes.Mol. Physiol. 8:215–234
Lauf, P.K. 1985. Passive K+Cl− fluxes in low K+ sheep erythrocytes: Modulation by A23187 and bivalent cations.Am. J. Physiol. 249:C271-C278
Lauf, P.K. 1985. K+:Cl− cotransport: Sulfhydryls, divalent cations, and the mechanism of volume activation in a red cell.J. Membrane Biol. 88:1–13
Lauf, P.K. 1987. Thiol-dependent passive K/Cl transport in sheep red cells: VII. Volume-independent freezing by iodoacetamide and sulfhydryl group heterogeneity.J. Membrane Biol. 98:237–246
Lauf, P.K., Adragna, J.C., Garay, R.P. 1984. Activation by N-ethylmaleimide of a latent K+−Cl− flux in human red blood cells.Am. J. Physiol. 246:C385-C390
Lauf, P.K., Perkins, C.M., Adragna, N.C. 1985. Cell volume and metabolic dependence of the N-ethylmaleimide activated K+Cl− flux in human red cells.Am. J. Physiol. 249:C124-C128
Lauf, P.K., Theg, B.E. 1980. A chloride dependent K+ flux induced by N-ethylmaleimide in genetically low K+ sheep and goat erythrocytes.Biochem. Biophys. Res. Commun. 92:1422–1428
Logue, P., Anderson, C., Kanik, C., Farquharson, B., Dunham, P.B. 1983. Passive potassium transport in LK sheep red cells. Modification by N-ethylmaleimide.J. Gen. Physiol. 81:861–885
Mannervik, B., Eriksson, S.A. 1974. Enzymatic reduction of mixed dissulfides and thiosulfate esters.In: Glutathione. L. Flohe et al., editors. pp. 120–132. Academic Press. New York, and G. Thieme, Stuttgart
Meister, A. 1983. Selective modification of glutathione metabolism.Science 220:471–477
Robillard, G.T., Konings, W.N. 1983. A hypothesis for the role of dithiol-disulfide interchange in solute transport and energy-transducing processes.Eur. J. Biochem. 127:597–604
Thornhill, W.G., Laris, P.C. 1984. KCl loss and cell shrinkage in the Ehrlich ascites tumor cell induced by hypotonic media, 2-deoxyglucose and propranolol.Biochim. Biophys. Acta 773:207–218
Tosteson, D.C., Hoffman, J.F. 1960. Regulation of cell volume by active cation transport in high and low potassium sheep red cells.J. Gen. Physiol. 44:169–194
Tucker, E.M., Kilgour, L. 1970. An inherited glutathione deficiency and a concomitant reduction in potassium concentration in sheep red cells.Experientia 26:203–204
Wiater, L.A., Dunham, P.B. 1983. Passive transport of K+ and Na+ in human red blood cells: Sulfhydryl binding agents and furosemide.Am. J. Physiol. 245:C348-C356
Zade-Oppen, A.M.M., Lauf, P.K. 1987. Interior and exterior pH sensitivity of ouabain-resistant potassium (K+) transport in low K− sheep red blood cells.J. Gen. Physiol. 90:44a
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lauf, P.K. Thiol-dependent K∶Cl transport in sheep red cells: VIII. Activation through metabolically and chemically reversible oxidation by diamide. J. Membrain Biol. 101, 179–188 (1988). https://doi.org/10.1007/BF01872833
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01872833