Summary
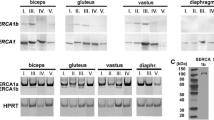
Four monoclonal antibodies against the calcium ATPase in sarcoplasmic reticulum (SR) of rabbit fast-twitch skeletal muscle were characterized using SDS-PAGE, Western blots and immunofluorescence. The ultrastructural distribution of the antigens was determined using post-embedding immunolabeling. The antibodies recognized the calcium ATPase in the SR but not in transverse (T-) tubule or plasma membranes. The antibody, D12, had the same binding affinity for the calcium ATPase from fast-twitch (rabbit sternomastoid) and slow-twitch (rabbit soleus) fibers and the affinity fell by 30% after fixation for electron microscopy in both types of muscle fiber. Ultrastructural studies revealed that the density of D12 antibody binding to the terminal cisternae membrane of extensor digitorum longus (edl) and sternomastoid fibers was on average seven times greater than in the slow-twitch soleus and semimembranosus fibers. Since the affinity of the ATPase for the antibody was the same in SR from fast- and slow-twitch muscles, the concentration of calcium ATPase in the terminal cisternae membrane of fast-twitch fibers was seven times greater than in slow-twitch fibers. This conclusion was supported by the fact that the concentration of calcium ATPase in light SR membranes was six times greater in SR from fast-twitch fibers than in SR from slow-twitch fibers. The results provide strong evidence that the different calcium accumulation rates in mammalian fast- and slow-twitch muscles are due to different concentrations of calcium ATPase molecules in the SR membrane.
Similar content being viewed by others
References
Banyard, M.R.C. 1984. A comparison of the expression of the DIDS-binding proteins from normal and tumorigenic human cells.Br. J. Cancer,50:809–814
Beringer, T. 1976. A freeze-fracture study of sarcoplasmic reticulum from fast and slow muscle of the mouse.Anat. Rec. 184:647–664
Bers, G., Garfin, D. 1985. Protein and nucleic acid blotting and immunochemical detection.Biotechniques 3:276–288
Bray, D.F., Rayns, D.G. 1976. A comparative freeze-etch study of the sarcoplasmic reticulum of avian fast and slow muscle fibres.J. Ultrastruct. Res. 57:251–259
Close, R. 1972. Dynamic properties of mammalian skeletal muscle.Physiol. Rev. 52:129–197
Damiani, E., Betto, R., Salvatiori, S., Volpe, P., Salviati, G., Margreth, A. 1981. Polymorphism of sarcoplasmic-reticulum adenosine triphosphatase of rabbit skeletal muscle.Biochem. J. 197:245–248
Davey, D.F., Wong, S.Y.P. 1980. Morphometric analysis of rat extensor digitorum longus and soleus muscles.Aust. J. Exp. Biol. Med. Sci. 58:393–404
DeFoor, P.H., Levitsky, D., Biryukova, T., Fleischer, S. 1980. Immunological dissimilarity of the calcium pump protein of skeletal and cardiac muscle sarcoplasmic reticulum.Arch. Biochem. Biophys. 200:196–205
Dulhunty, A.F., Dlutowski, M. 1979. Fibre types in red and white segments of rat sternomastoid muscle.Am. J. Anat. 156:51–62
Dulhunty, A.F., Gage, P.W. 1985. Excitation-contraction coupling and charge movement in denervated rat extensor digitorum longus and soleus muscles.J. Physiol. (London) 358:75–89
Dulhunty, A.F., Gage, P.W., Lamb, G.D. 1986. Differential effects of thyroid hormone on T-tubules and terminal cisternae in rat muscles: An electrophysiological and morphometric analysis.J. Mus. Res. Cell. Motil. 7:225–236
Dulhunty, A.F., Valois, A.A. 1983. Indentations in the terminal cisternae of amphibian and mammalian skeletal muscle fibres.J. Ultrastruct. Res. 84:34–49
Dux, L., Martonosi, A. 1984. Membrane crystals of Ca2+-ATPase in sarcoplasmic reticulum of fast and slow skeletal and cardiac muscles.Eur. J. Biochem. 141:43–49
Eisenberg, B.R. 1983. Quantitative ultrastructure of mammalian skeletal muscle.In: Handbook of Physiology. L.D. Peachey and R.H. Adrian, editors. pp. 73–112. American Physiology Society, Washington, D.C.
Eisenberg, B.R., Kuda, A.M. 1976. Discrimination between fibre populations in mammalian skeletal muscle by using ultrastructural parameters.J. Ultrastruct. Res. 54:76–88
Franzini-Armstrong, C.F. 1975. Membrane particles and transmission at the triad.Fed. Proc. 34:1382–1389
Heilmann, C., Brdiczka, D., Nickel, E., Pette, D. 1977. ATPase activities, Ca2+ transport and phosphoprotein formation in sarcoplasmic reticulum subfraction of fast and slow rabbit muscles.Eur. J. Biochem. 81:211–222
Heilmann, C., Müller, W., Pette, D. 1981. Correlation between ultrastructural and functional changes in sarcoplasmic reticulum during chronic stimulation of fast muscle.J. Membrane Biol. 59:143–149
Hidalgo, C., Gonzalez, M.E., Lagos, R. 1983. Characterization of the Ca2+- or Mg2+-ATPase of transverse tubule membranes isolated from rabbit skeletal muscle.J. Biol. Chem. 258:13937–13945
Jorgensen, A.D., Campbell, K.P. 1986. Monoclonal antibody to the Ca2++Mg2+-dependent ATPase of cardiac sarcoplasmic reticulum (SR) crossreacts with type I (slow) but not type II (fast) skeletal muscle.Biophys. J. 49:589P
Jorgensen, A.D., Kalnins, V., MacLennan, D.H. 1979. Localization of sarcoplasmic reticulum proteins in rat skeletal muscle by immunofluorescence.J. Cell. Biol. 80:372–384
Jorgensen, A.D., Shen, A.C.Y., MacLennan D.H., Tokuyasu, K.T. 1982. Ultrastructural localization of the Ca2++Mg2+-dependent ATPase of sarcoplasmic reticulum in rat skeletal muscle by immunoferritin labelling of ultrathin frozen sections.J. Cell Biol. 92:409–416
Kohler, G., Milstein, C. 1975. Continuous cultures of fused cells secreting antibody of a predefined specificity.Nature (London) 256:495–497
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature (London) 256:495–497
Lannergren, J. 1979. An intermediate type of muscle fibre inZenopus laevis.Nature (London) 279:254–256
Lannergren, J., Hoh, J.F.Y. 1984. Myosin isoenzymes in single muscle fibres ofXenopus laevis: Analysis of five different functional types.Proc. R. Soc. London B 222:401–408
Lannergren, J., Smith, R.S. 1966. Types of muscle fibres in toad skeletal muscle.Acta Physiol. Scand. 68:263–274
Maclennan, D.H., Brandl, C.J., Korczac, B., Green, N.M. 1985. Amino-acid sequence of a Ca2++Mg2+-dependent ATP'ase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence.Nature (London) 316:696–700
MacLennan, D.H., Seeman, P., Iles, G.H., Yip, C.C. 1971. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum.J. Biol. Chem. 246:2702–2710
Malouf, N., Meissner, G. 1979. Localization of a Mg2+- or Ca2+-activated (“basic”) ATPase in skeletal muscle.Exp. Cell Res. 122:233–250
Meissner, G., Fleischer, S. 1974. Dissociation and reconstitution of functional sarcoplasmic reticulum vescicles.J. Biol. Chem. 249:302–309
Mitchell, R.D., Saito, A., Palade, P., Fleischer, S. 1983. Morphology of isolated triads.J. Cell Biol. 96:1017–1029
Pette, D., Heilman, C. 1979. Some characteristics of sacroplasmic reticulum in fast- and slow-twitch muscles.Biochem. Soc. Trans. 581:765–766
Saito, A., Seiler, S., Chris, A., Fleischer, S. 1984. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle.J. Cell Biol. 99:875–885
Sreter, F.A., Gergely, J. 1964. Comparative studies of the Mg2+-activated ATPase activity and Ca2+-uptake of fractions of white and red muscle homogenates.Biochem. Biophys. Res. Commun. 16:438–443
Stewart, P.S., MacLennan, D.H. Shamoo, A.E. 1976. Isolation and characterization of tryptic fragments of the adenosine triphosphatase of sarcoplasmic reticulum.J. Biol. Chem. 251:712–719
Tada, M., Katz, A.M. 1982. Phosphorylation of the sarcoplasmic reticulum and sarcolemma.Annu. Rev. Physiol. 44:401–421
Tobin, H., Staehelin, T., Gordon, J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications.Proc. Natl. Acad. Sci. USA 76:4350–4353
Volpe, P., Damiani, E., Salviati, G., Margreth, A. 1982. Transitions in membrane composition during postnatal development of rabbit fast muscle.J. Musc. Res. Cell Motil. 3:213–230
Wendt, I.R., Gibbs, C.L. 1973. Energy production in rat extensor digitorum longus muscle.Am. J. Physiol. 224:1081–1086
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dulhunty, A.F., Banyard, M.R.C. & Medveczky, C.J. Distribution of calcium ATPase in the sarcoplasmic reticulum of fast- and slow-twitch muscles determined with monoclonal antibodies. J. Membrain Biol. 99, 79–92 (1987). https://doi.org/10.1007/BF01871228
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871228