Summary
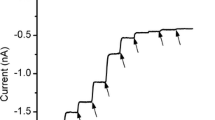
Using the dialysable detergent CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate), the tetrodotoxin-binding protein from the electroplax of the electric eel has been purified to a high degree of both chemical homogeneity and toxin-binding activity. On sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the best preparations showed only a single microheterogeneous band atM r approximately 260,000, despite attempts to visualize smaller bands by sample overloading. Upon dialysis, this material became incorporated into the membranes of small unilamellar vesicles, and in this form the purified protein exhibited tetrodotoxin-binding properties similar to the component in the original electroplax membrane. Furthermore, in the presence of activator neurotoxins the vesicles were able to accumulate isotopic sodium in a manner similar to that previously described for less active or less pure preparations of vesicles containing either mammalian or eel electroplax toxinbinding proteins. Quantitative consideration of the isotopic transport activity of this pure material, along with the high degree of purity of the protein, strongly suggests that the 260-kDa glycopeptide from electroplax is necessary and sufficient to account for the sodium channel function seen in these studies, and eliminates the possible involvement of smaller peptides in the channel phenomena observed.
Similar content being viewed by others
References
Agnew, W.S. 1984. Voltage-regulated sodium channels.Annu. Rev. Physiol. 46:517–530
Agnew, W.S., Levinson, S.R., Brabson, J.S., Raftery, M.A. 1978. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel fromElectrophorus electricus electroplax membranes.Proc. Natl. Acad. Sci. USA 75:2606–2610
Benzer, T.I., Raftery, M.A. 1972. Partial characterization of a tetrodotoxin-binding component from nerve membrane.Proc. Natl. Acad. Sci. USA 69:3634–3637
Bevington, P.R. 1969. Data Reduction and Error Analysis for the Physical Sciences. McGraw-Hill, New York
Catterall, W.A. 1986. Molecular properties of voltage-sensitive sodium channels.Annu. Rev. Biochem. 55:953–985
Duch, D.S., Levinson, S.R. 1987. Spontaneous opening at zero membrane potential of sodium channels from eel electroplax reconstituted into lipid vesicles.J. Membrane Biol. 98:57–68
Elmer, L.W., O'Brien, B.J., Nutter, T.J., Angelides, K.J. 1985. Physicochemical characterization of the alpha-peptide of the sodium channel from rat brain.Biochemistry. 24:8128–8137
Epstein, M., Racker, E. 1978. Reconstitution of carbamylcholine dependent sodium ion flux and desensitization of the acetylcholine receptor fromTorpedo californica.J. Biol. Chem. 19:6660–6662
Garty, H., Rudy, B., Karlish, S.J.D. 1983. A simple and sensitive procedure for measuring isotope fluxes through ion-specific channels in heterogeneous populations of membrane vesicles.J. Biol. Chem. 258:13094–13099.
Gasko, O.D., Knowles, A.F., Shertzer, H.G., Suolinna, E.M., Racker, E. 1976. The use of ion-exchange resins for studying ion transport in biological systems.Anal. Biochem. 72:57–65
Goldin, A.L., Snutch, T., Lubbert, H., Dowsett, A., Marshall, J., Auld, V., Downey, W., Fritz, H.C., Lester, H.A., Dunn, R., Catterall, W.A., Davidson, N. 1986. Messenger RNA coding for only the alpha subunit of the rat brain sodium channels is sufficient for expression of functional channels inXenopus oocytes.Proc. Natl. Acad. Sci. USA 83:7503–7507
Hartree, E.F. 1972. Determination of protein: A modification of the Lowry method that gives a linear photometric response.Anal. Biochem. 48:422–427
Hartshorne, R.P., Catterall, W.A. 1984. The sodium channels from rat brain: Purification and subunit composition.J. Biol. Chem. 259:1667–1675
Hartshorne, R.P., Keller, B.U., Talvenheimo, J.A., Catterall, W.A., Montal, M. 1985. Functional reconstitution of the purified brain sodium channel in planar lipid bilayers.Proc. Natl. Acad. Sci. USA 82:240–244
Hudson, C.S., Rash, J.E., Graham, W.F. 1979. Sample preparation for freeze-fracture.In: Freeze-Fracture: Methods, Artifacts, Interpretations. J.E. Rash and C.S. Hudson, editors. pp. 1–10. Raven, New York
Kagawa, Y., Racker, E. 1971. Partial resolution of the enzymes catalyzing oxidative phosphorylation.J. Biol. Chem. 246:5477–5487
Kraner, S.D., Tanaka, J.C., Barchi, R.L. 1985. Purification and functional reconstitution of the voltage-sensitive sodium channel from rabbit T-tubular membranes.J. Biol. Chem. 260:6341–6347
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature (London) 227:680–685
Levinson, S.R. 1975. The purity of tritiated tetrodotoxin as determined by bioassay.Phil. Trans. R. Soc. London B. 270:337–348
Levinson, S.R., Curatalo, C.J., Reed, J., Raftery, M.A. 1979. A rapid and precise assay for tetrodotoxin binding to detergent extracts of excitable tissues.Anal. Biochem. 99:72–84
Messner, D.J., Catterall, W.A. 1985. The sodium channel from rat brain. Separation and characterization of subunits.J. Biol. Chem. 260:10597–10604
Messner, D.J., Catterall, W.A. 1986. The sodium channel from rat brain. Role of the beta-1 and beta-2 subunits in saxitoxin binding.J. Biol. Chem. 261:211–215
Miller, J.A., Agnew, W.S., Levinson, S.R. 1983. Principle glycopeptide of the tetrodotoxin/saxitoxin binding protein fromElectrophorus electricus: Isolation and partial chemical and physical characterization.Biochemistry 22:462–470
Noda, M., Ikeda, T., Suzuki, H., Takeshima, H., Takahashi, T., Kuno, M., Numa, S. 1986. Expression of functional sodium channels from cloned cDNA.Nature (London) 322:826–828
Phillips, T.E., Boyne, A.S. 1984. Liquid nitrogen-bond quick freezing: Experiments with bounce-free delivery of cholinergic nerve terminals to a metal surface.J. Electr. Microsc. Techn. 1:9–29
Reed, J.K., Raftery, M.A. 1976. Properties of the tetrodotoxin binding component in plasma membranes isolated fromElectrophorus electricus.Biochemistry 15:944–953
Rosenberg, R.L., Tomiko, S.A., Agnew, W.S. 1984a. Reconstitution of neurotoxin-modulated ion transport by the voltageregulated sodium channel isolated from the electroplax ofElectrophorus electricus.Proc. Natl. Acad. Sci. USA 81:1239–1243
Rosenberg, R.L., Tomiko, S.A., Agnew, W.S. 1984b. Singlechannel properties of the reconstituted voltage-regulated Na channel isolated from the electroplax ofElectrophorus electricus.Proc. Natl. Acad. Sci. USA 81:5594–5598
Talvenheimo, J.A., Tamkun, M.M., Catterall, W.A. 1982. Reconstitution of neurotoxin-stimulated sodium transport by the voltage-sensitive sodium channel purified from rat brain.J. Biol. Chem. 257:11868–11871
Tamkun, M.M., Talvenheimo, J.A., Catterall, W.A. 1984. The sodium channel from rat brain: Reconstitution of neurotoxin-activated ion flux and scorpion toxin binding from purified components.J. Biol. Chem. 259:1676–1688
Tanaka, J.C., Eccleston, J.F., Barchi, R.L. 1983. Cation selectivity characteristics of the reconstituted sodium channel purified from rat skeletal muscle sarcolemma.J. Biol. Chem. 258:7519–7526
Tanaka, J.C., Furman, R.E., Barchi, R.L. 1986. Skeletal muscle sodium channels: Isolation and reconstitution.In: Ion Channel Reconstitution. C. Miller, editor, pp. 277–305. Plenum, New York
Tomiko, S.A., Rosenberg, R.L., Emerick, M.C., Agnew, W.S. 1986. Fluorescence assay for neurotoxin-modulated ion transport by the reconstituted voltage-activated sodium channel isolated from eel electric organ.Biochemistry 25:2162–2174
Weigele, J.B., Barchi, R.L. 1982. Functional reconstitution of the purified sodium channel protein from rat sarcolemma.Proc. Natl. Acad. Sci. USA 79:3651–3655
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duch, D.S., Levinson, S.R. Neurotoxin-modulated uptake of sodium by highly purified preparations of the electroplax tetrodotoxin-binding glycopeptide reconstituted into lipid vesicles. J. Membrain Biol. 98, 43–55 (1987). https://doi.org/10.1007/BF01871044
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871044