Summary
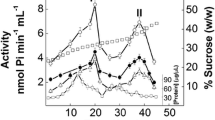
To characterize the molecular properties conveyed by the isoforms of the α subunit of Na,K-ATPase, the two major transepithelial transporting organs in the brine shrimp (Artemia salina), the salt glands and intestines, were isolated in pure form. The α isoforms were quantified by ATP-sensitive fluorescein isothiocyanate (FITC) labeling. The salt gland enzyme exhibits only the α1 isoform, whereas the intestinal enzyme exhibits both the α1 and the α2 isoforms. After 32 hours of development, Na,K-ATPase activity [in μmol Pi/mg protein/hr (1u)] in whole homogenates was 32±6 in the salt glands and 12±3 in the intestinal preparations (mean±sem). The apparent half-maximal activation constants (K 1/2) of the salt gland enzyme as compared to the intestinal enzyme were 3.7±0.6mm vs. 23.5±4mm (P<0.01) for Na+, 16.6±2.2mm vs. 8.29±1.5mm for K+ (P<0.01), and 0.87±0.8mm vs. 0.79±1.1mm for ATP (NS). The apparentK i's for ouabain inhibition were 1.1×10−4 m vs. 2×10−5 m, respectively. Treatment of whole homogenates with deoxycholic acid (DOC) produced a maximal Na,K-ATPase activation of 46% in the salt gland as compared to 23% in the intestinal enzyme. Similar differences were found with sodium dodecyl sulfate (SDS). The two distinct forms of Na,K-ATPase isolated from the brine shrimp differed markedly in three kinetic parameters as well as in detergent sensitivity. The differences inK 1/2 for Na+ and K+ are more marked than those reported for the mammalian Na,K-ATPase isoforms. These differences may be attributed to the relative abundances of the α subunit isoforms; other potential determinants (e.g. differences in membrane lipids), however, have not been investigated.
Similar content being viewed by others
References
Akera, T. 1981. Effects of cardiac glycosides on Na,K-ATPase.In: Handbook of Experimental Pharmacology. Vol. 56/1, Cardiac Glycosides. K. Greef, editor. pp. 287–336. Springer-Verlag, Heidelberg
Churchill, L., Hall, C., Peterson, G., Ruoho, A.E., Hokin, L.E. 1984. Photoaffinity labeling of the ouabain binding site in Na,K-ATPase in developing brine shrimp.J. Exp. Zool. 231:343–350
Conte, F.P. 1984. Structure and function of the crustacean larval salt gland.Int. Rev. Cytol. 91:45–104
Cortas, N., Walser, M. 1971. (Na++K+-activated ATPase in isolated mucosal cells of toad bladder.Biochim. Biophys. Acta 249:181–187
Doucet, A., Barlet, C. 1986. Evidence for differences in the sensitivity to ouabain of Na,K-ATPase along the nephrons of rabbit kidney.J. Biol. Chem. 261:993–995
Fambrough, D.M., Bayne, E.K. 1983. Multiple forms of Na,K-ATPase in the chicken. Selective detection of the major nerve, skeletal muscle, and kidney form by a monoclonal antibody.J. Biol. Chem. 258:3926–3935
Geny, G., Paraf, A., Fedon, Y., Charlemagne, D. 1982. Characterization of a β-actinin-like protein in purified non-muscle cell membranes. Its activity on (Na++K+)-ATPase.Biochim. Biophys. Acta 692:345–354
Jorgensen, P.L. 1974. Purification and characterization of Na,K-ATPase: III. Purification from the outer medulla of the mammalian kidney after selective removal of membrane components by deoxycholate.Biochim. Biophys. Acta 356:36–47
Kazazoglou, T., Renaud, J.F., Rosi, B., Lazdunski, M. 1983. Two classes of ouabain receptors in chick ventricular cardiac cells and their relation to Na,K-ATPase inhibition, intracellular Na+ accumulation, Ca2+ influx and cardiotonic effect.J. Biol. Chem. 258:12163–12170
Koch, K.S., Leffert, H.L. 1980. Growth control of differentiated adult rat hepatocytes in primary culture.Ann. N.Y. Acad. Sci. 349:111–227
Lane, L.K., Copenhaver, J.H., Lindenmayer, G.E., Schwartz, A. 1973. Purification and characterization of, and3H-ouabain binding to the transport adenosine triphosphatase from outer medulla of canine kidney.J. Biol. Chem. 248:7197–7200
Leffert, H.L., Koch, K.S. 1980. Ionic events at the membrane initiate rat liver regeneration,Ann. N.Y. Acad. Sci. 339:201–215
Lowry, O.H., Rosenborough, N.J., Farr, A.L., Randall, R.J. 1951. Protein measurement with the Folin reagent.J. Biol. Chem. 193:265
Lowy, R.J., Conte, F.P. 1985. Isolation and functional characterization of crustacean larval salt gland.Am. J. Physiol. 248:R702-R708
Lytton, J. 1985. Insulin affects the sodium affinity of the rat adipocyte (Na+,K+)-ATPase.J. Biol. Chem. 260:10075–10080
Lytton, J., Lin, J.C., Guidotti, G. 1985. Identification of two molecular forms of (Na+,K+)-ATPase in rat adipocytes. Relation to insulin stimulation of the enzyme.J. Biol. Chem. 260:1177
Matsuda, T., Iwata, H., Cooper, J.R. 1984. Specific inactivation of α(+) molecular form of (Na++K+)-ATpase by pyrithiamin.J. Biol. Chem. 259:3858
Morohashi, M., Kawamura, M. 1984. Solubilization and purification ofArtemia salina Na,K-ATPase and NH2-terminal amino acid sequence of its larger subunit.J. Biol. Chem. 259:14928–14934
Muallem, S., Karlish, S.J.D. 1983. Catalytic and regulatory ATP-binding sites of the red cell Ca2+ pump studied by irreversible modification with fluorescein isothiocyanate.J. Biol. Chem. 258:169–175
Peterson, G.L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable.Anal. Biochem. 83:346–356
Peterson, G.L., Churchill, L., Fisher, J., and Hokin, L. 1982. Structure and biosynthesis of Na,K-ATPase in developing brine shrimp nauplii.Ann. N.Y. Acad. Sci. 42:185–206
Peterson, G.L., Ewing, R.D., Hootman, S.R., Conte, F.P. 1978. Large scale partial purification, and molecular and kinetic properties of Na,K-ATPase fromArtemia salina nauplii.J. Biol. Chem. 253:4762–4770
Pressley, T.A., Edelman, I.S. 1986. Reduced ouabain inhibition of Na,K-ATPase in cultured cell recipients of the ouabain-resistance gene.J. Biol. Chem. 261:9779–9786
Pressley, T.A., Haber, R.S., Loeb, J., Edelman, I.S., Ismail-Beigi, F. 1986. Stimulation of Na,K-activated adenosine triphosphatase and active transport by low external K+ in rat liver cell line.J. Gen. Physiol. 87:591–606
Resh, M.D. 1982. Development of insulin responsiveness of the glucose transporter and the (Na+,K+)-adenosine triphosphatase during in vitro adipocyte differentiation.J. Biol. Chem. 257:6978–6986
Salon, J., Cortas, N., Edelman, I.S. 1989. Isoforms of Na,K-ATPase inArtemia salina: Detection by FITC binding and time course.J. Membrane Biol. 108:177–186
Shull, G.E., Greeb, J., Lingrel, J.B. 1986. Molecular cloning of three distinct forms of the Na+,K+-ATPase α-subunit from rat brain.Biochemistry 25:8125–8132
Shull, M., Lingrel, J.B. 1987. Multiple genes encode the human Na,K-ATPase catalytic subunit.Proc. Natl. Acad. Sci. USA 84:4039–4043
Siegel, G.J., Desmond, T., Ernest, S.A. 1986. Immunoreactivity and ouabain-dependent phosphorylation of Na,K-ATPase catalytic subunit doublets.J. Biol. Chem. 261:13768–13776
Skou, J.C. 1988. The Na,K-pump.Meth. Enzymol. 156:1–25
Specht, S.C., Sweadner, K.J. 1984. Two different Na,K-ATPases in the optic nerve: Cells of origin and axonal transport.Proc. Natl. Acad. Sci. USA 81:1234–1238
Sverdlov, E.D., Monastyrskaya, G.S., Broude, N.E., Ushkayov, Y.A., Allikmets, R.L. 1987. The family of human Na,K-ATPase genes. At least 5 genes and/or pseudogenes homologous to α subunit.Dokl. Acad. Nauk. SSSR 294:734–738
Sweadner, K.J. 1979. Two molecular forms of (Na++K+)-stimulated ATPase in brain. Separation, and difference in affinity for strophanthidin.J. Biol. Chem. 354:6060–6067
Sweadner, K.J. 1985. Enzymatic Properties of separated isozymes of the Na,K-ATPase. Substrate affinities, kinetic cooperativity and ion transport stoichiometry.J. Biol. Chem. 260:11508–11513
Author information
Authors and Affiliations
Additional information
During the tenure of an Educational Commission For Foreign Medical Graduates Visiting Associate Professorship.
Rights and permissions
About this article
Cite this article
Cortas, N., Arnaout, M., Salon, J. et al. Isoforms of Na,K-ATPase inArtemia salina: II. Tissue distribution and kinetic characterization. J. Membrain Biol. 108, 187–195 (1989). https://doi.org/10.1007/BF01871029
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871029