Summary
The voltage-dependence of channel formation by alamethicin and its natural analogues can be described by a dipole flip-flop gating model, based on electric field-induced transbilayer orientational movements of single molecules. These field-induced changes in orientation result from the large permanent dipole moment of alamethicin, which adopts α-helical conformation in hydrophobic medium. It was, therefore, supposed that the only structural requirement for voltage-dependent formation of alamethicin-type channels might be a rigid lipophilic helical segment of minimum length.
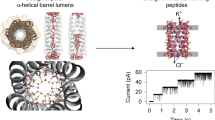
In order to test this hypothesis we synthesized a family of lipophilic polypeptides—Boc-(Ala-Aib-Ala-Aib-Ala) n -OMe,n=1–4—which adopt α-helical conformation forn=2–4 and studied their interaction with planar lipid bilayers. Surprisingly, despite their large difference in chain length, all four polypeptides showed qualitatively similar behavior. At low field strength of the membrane electric field these polypeptides induce a significant, almost voltage-independent increase of the bilayer conductivity. At high field strength, however, a strongly voltage-dependent conductance increase occurs similar to that observed with alamethicin. It results from the opening of a multitude of ion translocating channels within the membrane phase.
The steady-state voltage-dependent conductance depends on the 8th–9th power of polypeptide concentration and involves the transfer of 4–5 formal elementary charges. From the power dependences on polypeptide concentration and applied voltage of the time constants in voltage-jump current-relaxation experiments, it is concluded that channels could be formed from preexisting dodecamer aggregates by the simultaneous reorientation of six formal elementary charges. Channels exhibit large conductance values of several nS, which become larger towards shorter polypeptide chain length. A mean channel diameter of 19 Å is estimated corresponding roughly to the lumen diameter of a barrel comprised of 10 α-helical staves. Similar to experiments with the N-terminal Boc-derivative of alamethicin we did not observe the burst sequence of nonintegral conductance steps typical of natural (N-terminal Ac-Aib)-alamethicin. Saturation in current/voltage curves as well as current inactivation in voltage-jump current-relaxation experiments are found. This may be understood by assuming that channels are generated as dodecamers but, while reaching the steady state, reduce their size to that of an octamer or nonamer. We conclude that the overall behavior of these synthetic polypeptides is very similar to that of alamethicin. They exhibit the same concentration and voltage-dependences but lack the stabilizing principle of resolved channel states characteristic of alamethicin.
Similar content being viewed by others
References
Andersen, O.S., Muller, R.U. 1982. Monazomycin-induced single channels: I. Characterization of the elementary conductance events.J. Gen. Physiol. 80:403–426
Boheim, G. 1974. Statistical analysis of alamethicin channels in black lipid membranes.J. Membrane Biol. 19:277–303
Boheim, G., Hanke, W., Eibl, H. 1980. Lipid phase transition in planar bilayer membrane and its effect on carrier- and poremediated ion transport.Proc. Natl. Acad. Sci. USA 77:3403–3407
Boheim, G., Hanke, W., Jung, G. 1983. Alamethicin pore formation: Voltage-dependent flip-flop of α-helix dipoles.Biophys. Struct. Mechan. 9:181–191
Boheim, G., Hanke, W., Überschär, S., Eibl, H. 1982. Alamethicin pore formation in planar bilayers above and below lipid phase transition temperature.In: Transport in Biomembranes: Model Systems and Reconstitution. R. Antolini, et al., editors, pp. 135–143. Raven, New York
Boheim, G., Janko, K., Leibfritz, D., Ooka, T., König, W.A., Jung, G. 1976. Structural and membrane modifying properties of suzukacillin, a peptide atibiotic related to alamethicin. Part B: Pore formation in black lipid films.Biochim. Biophys. Acta 433:182–199
Boheim, G., Kolb, H.A. 1978. Analysis of the multi-pore system of alamethicin in a lipid membrane: I. Voltage-jump current-relaxation experiments.J. Membrane Biol. 38:99–150
Bosch, R. 1984. Röntgenstrukturanalysen und vergleichende Studies von Alamethicin-Segmenten mit β-Turns, 3 110 - und 3 ′10 -, sowie α-Helices. Ph.D. Thesis. University of Tübingen, Tübingen
Bosch, R., Jung, G., Schmitt, H., Sheldrick, G.M., Winter, W. 1984. Peptide structures of the alamethicin sequence: The C-terminal α/310-helical nonapeptide and two pentapeptides with opposite 310-helicity.Angew. Chem. Int. Ed. Engl. 23:450–453
Bosch, R., Jung, G., Schmitt, H., Winter, W. 1985a. Crystal structure of the α-helical undecapeptide Boc-l-Ala-Aib-Ala-Aib-Ala-Glu(OBzl)-Ala-Aib-Ala-Aib-Ala-OMe.Biopolymers 24:961–978
Bosch, R., Jung, G., Schmitt, H., Winter, W. 1985b. Crystal structure of Boc-Leu-Aib-Pro-Val-Aib-Aib-Glu(OBzl)-Gln-Phl·H2O, the C-terminal nonapeptide of the voltage-dependent ionophore alamethicin.Biopolymers 24:979–999
Bosch, R., Jung, G., Winter, W. 1983. Structure of the 310-helical pentapeptide Boc-Aib-l-Ala-Aib-l-Ala-Aib-OMe ·2 H2O.Acta Crystallogr. Sect. C 39:776–778
Brückner, H., Graf, H., Bokel, M. 1984. Paracelsin: Characterization by NMR spectroscopy and circular dichroism, and hemolytic properties of a peptaibol antibiotic from the cellulolytically active moldTrichoderma reesei. Part B.Experientia 40:1189–1197
Brückner, H., Jung, G. 1982. Synthesis ofl-Pro-Leu-Aib-Aib-Gln-Valol and proof of identity with the isolated C-terminal fragment of trichotoxin A40.Liebigs Ann. Chem. 1982:1677–1699
Brückner, H., König, W.A., Aydin, M., Jung, G. 1985. Trichotoxin A40. Purification by counter current distribution and sequencing of isolated fragments.Biochim. Biophys. Acta 827:51–62
Brückner, H., Nicholson, G.J., Jung, G., Kruse, K., König, W.A. 1980. Gas chromatographic determination of the configuration of isovaline in antiamoebin, samarosporin (emerimicin IV), stilbellin, suzukacillins and trichotoxins.Chromatographia 13:209–214
Brückner, H., Przybylski, M. 1984. Isolation and structural characterization of polypeptide antibiotics of the peptaibol class by HPLC with FD and FAB mass spectrometry.J. Chromat. 296:263–275
Butters, T., Hütter, P., Jung, G., Pauls, N., Schmitt, H., Sheldrick, G.M., Winter, W. 1981. On the structure of the helical N-terminus in alamethicin-α-helix or 310-helix?Angew. Chem. Int. Ed. Engl. 20:889–890
Davis, P.J., Fleming, B.D., Coolbear, K.P., Keough, K.M.W. 1981. Gel to liquid-crystalline transition temperatures of water dispersions of two pairs of positional isomers of unsaturated mixed-acid phosphatidylcholines.Biochemistry 20:3633–3636
Edmonds, D.T. 1980. Membrane ion channels and ionic hydration energies.Proc. R. Soc. London B 211:51–62
Eisenberg, M., Hall, J.E., Mead, C.A. 1973. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes.J. Membrane Biol. 14:143–176
Finkelstein, A., Holz, R. 1973. Aqueous pores creasted in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B.In: Membranes. Vol. 2. Lipid Bilayers and Antibiotics. G. Eisenman, editor, pp. 377–408. M. Dekker, New York
Fox, R.O., Richards, F.M. 1982. A voltage-gated ion channel model inferred from crystal structure of alamethicin at 1.5 Å resolution.Nature (London) 300:325–330
Gordon, L.G.M., Haydon, D.A. 1972. The unit conductance channel of alamethicin.Biochim. Biophys. Acta 255:1014–1018
Hall, J.E., Vodyanoy, I., Balasubramanian, T.M., Marshall, G.R. 1984. Alamethicin—A rich model for channel behavior.Biophys. J. 45:233–247
Hanke, W., Boheim, G. 1980. The lowest conductance state of the alamethicin pore.Biochim. Biophys. Acta 596:456–462
Hanke, W., Methfessel, C., Wilmsen, H.-U., Katz, E., Jung, G., Boheim, G. 1983. Melittin and a chemically modified trichotoxin form alamethicin-type multi-state pores.Biochim. Biophys. Acta 727:108–114
Heyer, E.J., Muller, R.U., Finkelstein, A. 1976. Inactivation of monazomycin-induced voltage-dependent conductance in thin lipid membranes: II. Inactivation produced by monazomycin transport through the membrane.J. Gen. Physiol. 67:731–748
Hol, W.G.J. 1985. The role of the α-helix dipole in protein function and structure.Prog. Biophys. Molec. Biol. 45:149–195
Hol, W.G.J., Duijnen, P.T. van, Berendsen, H.J.C. 1978. The α-helix dipole and the properties of proteins.Nature (London) 273:443–446
Hol, W.G.J., Halie, L.M., Sander, C. 1981. Dipoles of the α-helix and β-sheet: Their role in protein folding.Nature (London) 294:532–536
Jung, G., Becker, G., Schmitt, H., Voges, K.-P., Boheim, G., Griesbach, S. 1983a. Voltage-gated membrane pores are formed by a flip-flop of α-helical polypeptides.In: Peptides, Structure and Function. V.J. Hruby, and D.H. Rich, editors. pp. 491–494. Pierce Chemical Co., Rockford, Ill.
Jung, G., Bosch, R., Katz, E., Schmitt, H., Voges, K.-P., Winter, W. 1983b. Stabilizing effects of 2-methylalanine residues on β-turns and α-helices.Biopolymers 22:241–246
Jung, G., Dubischar, N., Leibfritz, D. 1975. Solvent and temperature induced conformational changes of alamethicin, a13C NMR and circular dichroism study.Eur. J. Biochem. 54:395–409
Jung, G., Katz, E., Schmitt, H., Voges, K.-P., Menestrina, G., Boheim, G. 1983c. Conformational requirements for the potential dependent pore formation of the peptide antibiotics alamethicin, suzukacillin and trichotoxin.In: Physical Chemistry of Transmembrane Ion Motions. G. Spach, editor. pp. 349–357. Elsevier, Amsterdam
Katz, E., Aydin, M., Lucht, N., König, W.A., Ooka, T., Jung, G. 1985. Sequence and conformation of suzukacillin A.Liebigs Ann. Chem. 1985:1041–1062
Kleinberg, M.E., Finkelstein, A. 1984. Single-length and double-length channels formed by nystatin in lipid bilayer membranes.J. Membrane Biol. 80:257–269
Marty, A., Finkelstein, A. 1975. Pores formed in lipid bilayer membranes by nystatin. Differences in its one-sided and two-sided action.J. Gen. Physiol. 65:515–526
Mathew, M.K., Balaram, P. 1983a. Alamethicin and related membrane channel forming polypeptides.Mol. Cell. Biochem. 50:47–65
Mathew, M.K., Balaram, P. 1983b. A dipole helix model for alamethicin and related transmembrane channels.FEBS Lett. 157:1–5
McIntosh, T.J., Ting-Beall, H.P., Zampighi, G. 1982. Alamethicin induced changes in lipid bilayer morphology.Biochim. Biophys. Acta 685:51–60
Montal, M., Mueller P. 1972. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties.Proc. Natl. Acad. Sci. USA 69:3561–3566
Mueller, P. 1976. Molecular aspects of electrical excitation in lipid bilayers and cell membranes.Horizons Biochem. Biophys. 2:230–284
Mueller, P., Rudin, D.O. 1968. Action potentials induced in bimolecular lipid membranes.Nature (London) 217:713–719
Muller, R.U., Andersen, O.S. 1982. Monazomycin-induced single channels. II. Origin of the voltage dependence of the macroscopic conductance.J. Gen. Physiol. 80:427–449
Muller, R.U., Finkelstein, A. 1972. Voltage-dependent conductance induced in thin lipid membranes by monazomycin.J. Gen. Physiol. 60:263–284
Nakayama, H., Furihata, K., Seto, H., Otake, N. 1981. Structure of monazomycin, a new ionophorous antibiotic.Tetrahedron Lett. 22:5217–5220
Rinehart, K.L., Cook, J.C., Meng, H., Olson, K.L., Pandey, R.C. 1977. Mass spectrometric determination of molecular formulas for membrane-modifying antibiotics.Nature (London) 269:832–833
Rizzo, V., Schwarz, G., Voges, K.-P., Jung, G. 1985. Molecular shape and dipole moment of alamethicin-like synthetic peptides.Eur. Biophys. J. 12:67–73
Robinson, R.A., Stokes, R.H. 1959. Electrolyte Solutions. Butterworth, London
Roy, G. 1975. Properties of the conductance induced in lecithin bilayer membranes by alamethicin.J. Membrane Biol. 24:71–85
Schmitt, H., Jung, G. 1985a. Total synthesis of the α-helical eicosapeptide antibiotic alamethicin.Liebigs Ann. Chem. 1985:321–344
Schmitt, H., Jung, G. 1985b.13C NMR spectroscopic control of the synthesis of Alamethicin F30 and its segments.Liebigs Ann. Chem. 1985:345–364
Schmitt, H., Winter, W., Bosch, R., Jung, G. 1982. The α-helical conformation of the undecapeptide Boc-l-Ala-(Aib-Ala)2-Glu(OBzl)-Ala-(Aib-Ala)2-OMe: Synthesis, X-ray crystal structure, and conformation in solution.Liebigs Ann. Chem. 1982:1304–1321
Schwarz, G., Savko, P. 1982. Structural and dipolar properties of the voltage dependent pore former alamethicin in octanol/dioxane.Biophys. J. 39:211–219
Schwarz, G., Savko, P., Jung, G. 1983. Solvent dependent structural features of the membrane active peptide trichotoxin A40 as reflected in its dielectric dispersion.Biochim. Biophys. Acta 718:419–428
Spach, G., Trudelle, Y., Heitz, F. 1983. Peptides as channelmaking ionophores: Conformational aspects.Biopolymers 22:403–407
Urry, D.W., Bradley, R.J., Ohnishi, T. 1978. Characterization of a synthetic, voltage-dependent, cation-selective transmembrane channel.Nature (London) 274:382–383
Vodyanoy, I., Hall, J.E., Balasubramanian, T.M., Marshall, G.R. 1982. Two purified fractions of alamethicin have different conductance properties.Biochim. Biophys. Acta 684:53–58
Voges, K.-P. 1985. Zur Eintauchtiefe helikaler Tryptophanyl-Heneikosapeptide in Membranen: Synthese, NMR, CD und Fluoreszenz. Thesis, University of Tübingen, Tübingen
Yantorno, R.E., Takashima, S., Mueller, P. 1982. Dipole moment of alamethicin as related to voltage-dependent conductance in lipid bilayers.Biophys. J. 38:105–110
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Menestrina, G., Voges, KP., Jung, G. et al. Voltage-dependent channel formation by rods of helical polypeptides. J. Membrain Biol. 93, 111–132 (1986). https://doi.org/10.1007/BF01870804
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01870804