Summary
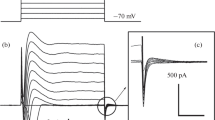
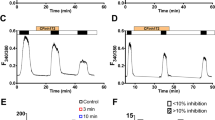
The effects of furosemide on the chloride-dependent short-circuit current across the toad ciliary epithelium were examined. Under control conditions, the short-circuit current obeyed Michaelis-Menten kinetics against medium chloride concentration, the Michaelis constant (K m ) for chloride being 90mm and the maximal short-circuit current (V max) 128 μA/cm2. Furosemide added to the aqueous side of the epithelium rapidly reduced the short-circuit current; the effect was reversible. The effect of furosemide addition to the stromal side was much smaller and slower than that from the aqueous side. The dose-dependent range of furosemide action was from 0.1 μm to 1mm with 50% inhibition occurring at about 3 μm. Line-weaver-Burk plot of the short-circuit current against the chloride concentration showed that furosemide decreased the value ofV max and increased theK m ; the inhibition being of mixed type. A Hill plot of the dose-response curve yielding a slope of unity suggested one furosemide molecule combines with one chloride transport site. Probenecid, a competitive inhibitor of organic acid transport, reduced the effects of furosemide significantly when added simultaneously. The involvement of organic acid transport system in the mechanism of furosemide action on chloride transport was suggested.
Similar content being viewed by others
References
Becker, B. 1960. The transport of organic anions by the rabbit eye. 1.In vitro iodopyracet (diodrast) accumulation by ciliary body-iris preprations.Am. J. Ophthalmol. 50:862
Bindslev, N., Tormey, J.McD., Wright, E.M. 1974. The effects of electrical and osmotic gradients on lateral intercellular spaces and membrane conductance in a low resistance epithelium.J. Membrane Biol. 19:357
Brazy, P.C., Gunn, R.B. 1976. Furosemide inhibition of chloride transport in human red blood cells.J. Gen. Physiol. 68:583
Burg, M.B. 1976. Tubular chloride transport and the mode of action of some diuretics.Kidney Int 9:189
Burg, M.B., Green, N. 1973. Function of the thick ascending limb of Henle's loop.Am. J. Physiol. 224:659
Burg, M.B., Stoner, L., Cardinal, J., Green, N. 1973. Furosemide effect on isolated perfused tubules.Am. J. Physiol. 225:119
Candia, O.A. 1973. Short-circuit current related to active transport of chloride in frog cornea: Effects of furosemide and ethacrynic acid.Biochim. Biophys. Acta 298:1011
Deetjen, P. 1966. Micropuncture studies on site and mode of diuretic action of furosemide.Ann. N.Y. Acad. Sci. 139:408
Deuticke, B., Gerlach, E. 1967. Beeinflussung von Form und Phosphat-permeabilität menschlicher Erythrocyten durch Hämolysine, Benzol-derivate und pharmakologisch aktive Substanzen.Klin. Wochenschr. 45:977
Dixon, M., Webb, E.C. 1964. Enzymes. p. 316. Longmans, London
Ferguson, D.R., Twite, B.R. 1975. The effect of diuretics on Na+−K+-ATPase and c-AMP levels in toad bladder epithelial cells.Naunyn Schmiedeberg's Arch. Pharmacol. 287:111
Field, M. 1977. Some speculations on the coupling between sodium and chloride transport processes in mammalian and teleost intestine.In: Membrane Transport Processes. J.F. Hoffman, editor. Vol. 1, p. 277. Raven Press, New York
Forbes, M., Becker, B. 1960. The transport of organic anions by the rabbit eye. II.In vivo transport of iodopyracet (diodrast).Am. J. Ophthalmol. 50:867
Frizzell, R.A., Heintze, K. 1979. Electrogenic chloride secretion by mammalian colon.In: Mechanisms of Intestinal Secretion. H.J. Binder, editor. p. 101. Alan R. Liss, New York
Ginzburg, S.G., Hogg, J. 1967. What does a short-circuit current measure in biological systems?J. Theor. Biol. 14:316
Goldstein, A., Aronow, L., Kalman, S.M. 1974. Principles of Drug Action: The Basis of Pharmacology. p. 82. John Wiley, New York
Guelrud, M., Rudick, J., Janowitz, H.D. 1972. Effects of some inhibitors of sodium transport (adenosine triphosphatase inhibitors) on pancreatic secretion.Gastroenterology 62:540
Holland, M.G. 1970. Chloride ion transport in the isolated ciliary body. II. Ion substitution experiments.Invest. Ophthalmol. 9:30
Holland, M.G., Gipson, C.C. 1970. Chloride ion transport in the isolated ciliary body.Invest. Ophthalmol. 9:20
Hook, J.B., Williamson, H.E. 1965a. Influence of probenecid and alterations in acid-base balance of the saluretic activity of furosemide.J. Pharmacol. Exp. Ther. 149:404
Hook, J.B., Williamson, H.E. 1965b. Lack of correlation between natriuretic activity and inhibition of renal Na−K-activated ATPase.Proc. Soc. Exp. Biol. Med. 120:358
Humphreys, M.H. 1976. Inhibition of NaCl absorption from perfused rat ileum by furosemide.Am. J. Physiol. 230:1517
Rocha, A.S., Kokko, J.P. 1973. Sodium chloride and water transport in the medullary thick ascending limb of Henle: Evidence for active chloride transport.J. Clin. Invest. 52:612
Sachs, J. 1971. Ouabain-insensitive sodium movements in human red blood cells.J. Gen. Physiol. 57:259
Saito, Y., Watanabe, T. 1979. Relationship between short-circuit current and unidirectional fluxes of Na and Cl across the ciliary epithelium of the toad: Demonstration of active Cl transport.Exp. Eye Res. 28:71
Schultz, S.G. 1979. Chloride transport by gastrointestinal epithelia: An overview.In Mechanisms of Intestinal Secretion. H.J. Binder, editor. p. 93. Alan R. Liss, New York
Ussing, H.H., Zerahn, K. 1951. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin.Acta Physiol. Scand. 23:110
Watanabe, T., Saito, Y. 1978. Characteristics of ion transport across the isolated ciliary epithelium of the toad as studied by electrical measurements.Exp. Eye Res. 27:215
Weiner, J.M., Washington, J.A., Mudge, G.H. 1960. On the mechanism of action of probenecid on renal tubular secretion.Bull. Johns Hopkins Hosp. 106:333
Wright, E.M. 1974. Active transport of iodide and other anions across the choroid plexus.J. Physiol. (London) 240:535
Zadunaisky, J.A. 1966. Active transport of chloride in frog cornea.Am. J. Physiol. 211:506
Zadunaisky, J.A. 1972. Sodium activation of chloride transport in the frog cornea.Biochim. Biophys. Acta 282:255
Author information
Authors and Affiliations
Additional information
Department of Ophthalmology.
Rights and permissions
About this article
Cite this article
Saito, Y., Itoi, K., Horiuchi, K. et al. Mode of action of furosemide on the chloride-dependent short-circuit current across the ciliary body epithelium of toad eyes. J. Membrain Biol. 53, 85–93 (1980). https://doi.org/10.1007/BF01870577
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01870577