Summary
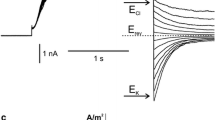
A stopped-flow spectrophotometric technique was used to study the kinetics of Ca flux into ciliary membrane vesicles fromParamecium tetraurelia wild-type and several ‘pawn’ mutants with defective Ca conductances. 15mm Arsenazo III was used as metallochromic indicator and as intravesicular Ca trap. The absolute amount of Ca-permeable vesicles was significantly reduced in preparations from the ‘pawn’ mutants compared to wild-type. However, influx kinetics were identical for vesicles from wild-type and ‘pawn’ mutantParamecia when the fraction of Ca-permeable vesicles was taken into account. Ca influx was rapid with a time constant of about 1.5 sec and an initial saturation rate of arsenazo III of about 50%/single vesicle ×sec−1. Ca influx rates were half-maximal at approximately 20 μm Ca. Comparisons of Ba toxicity tested with a behavioral assay, Ca inward conductances under voltage-clamp conditions and Ca influx kinetics between wild-type and the ‘pawn’ mutants pwA (d4-94), leaky pwB (d4-96) and the double mutant pwA/pwB indicated that Ca transport in all types of ciliary membrane vesicles occurred through similar Ca gates.
Similar content being viewed by others
References
Adoutte, A., Ramanathan, R., Lewis, R.M., Dute, R.R., Ling, K.-Y., Kung, C., Nelson, D.L. 1980. Biochemical studies of the excitable membrane ofParamecium tetraurelia. III. Proteins of cilia and ciliary membranes.J. Cell Biol. 84:717–738
Andrews, D., Nelson, D.L. 1979. Biochemical studies of the excitable membrane ofParamecium tetraurelia. II. Phospholipids of ciliary and other membranes.Biochim. Biophys. Acta 550:174–187
Bauer, P.J. 1981. Affinity and stoichiometry of calcium binding by arsenazo III.Anal. Biochem. 110:61–72
Brown, H.M., Rydquist, B. 1981. Arsenazo III-Ca. Effect of pH, ionic strength, and arsenazo III concentration on equilibrium binding evaluated with Ca2+ ion-sensitive electrodes and absorbance measurements.Biophys. J. 36:117–137
Browning, J.L., Nelson, D.L. 1976. Biochemical studies of the excitable ciliary membrane ofParamecium tetraurelia. I. Ca2+ fluxes across resting and excited membrane.Biochim. Biophys. Acta 448:338–351
Dorogi, P.L., Neumann, E. 1981. Spectrophotometric determination of reaction stoichiometry and equilibrium constants of metallochromic indicators. II. The Ca2+-arsenazo III complexes.Biophys. Chem. 13:125–131
Dunlap, K. 1977. Localization of calcium channels inParamecium caudatum.J. Physiol. (London) 271:119–133
Eckert, R., Brehm, P. 1979. Ionic mechanisms of excitation inParamecium.Annu. Rev. Biophys. Bioeng. 8:352–382
Hagiwara, S. 1981. General properties of gated calcium transport.In: The Mechanism of Gated Calcium Transport across Biological Membranes. S.T. Ohnishi and M. Endo, editors. pp. 3–7. Academic Press, New York
Hagiwara, S., Byerly, W.L. 1981. Calcium channels.Annu. Rev. Neurosci. 4:69–125
Kaneshiro, E.S., Beischel, L.S., Merkel, S.J., Rhoads, D.E. 1979. The fatty acid composition ofParamecium aurelia cells and cilia: Changes with culture age.J. Protozool. 26:147–158
Kung, C., Chang, S.Y., Satow, Y., Houten, J. van, Hansma, H. 1975. Genetic dissection of behavior inParamecium.Science 188:898–904
Ling, K.-Y., Kung, C. 1980. Ba2+ influx measures the duration of membrane excitation inParamecium.J. Exp. Biol. 84:73–87
Machemer, H., Ogura, A. 1979. Ionic conductances of membranes in ciliated and deciliatedParamecium.J. Physiol. (London) 29:49–60
Naitoh, Y., Kaneko, H. 1972. ATP-reactivated Triton-extracted models ofParamecium: Modification of ciliary movement by calcium ions.Science 176:523–524
Oertel, D., Schein, S.J., Kung, C. 1977. Separation of membrane currents using aParamecium mutant.Nature (London) 268:120–124
Ogura, A., Takahashi, K. 1976. Artificial deciliation causes loss of calcium-dependent responses inParamecium.Nature (London) 264:170–172
Peyer, J.E. de, Deitmer, J.W. 1980. Divalent cations as charge carriers during two functionally different membrane currents in the ciliateStylonychia.J. Exp. Biol. 88:73–89
Rhoads, D.E., Kaneshiro, E.S. 1979. Characterization of phospholipids fromParamecium tetraurelia cells and cilia.J. Protzool. 26:329–338
Saimi, Y., Kung, C. 1980. A Ca-induced Na-current inParamecium.J. Exp. Biol. 88:305–325
Saimi, Y., Kung, C. 1982. Are ions involved in the gating of calcium channels?Science 218:153–155
Satow, Y., Kung, C. 1979. Voltage sensitive Ca-channels and the transient inward current inParamecium tetraurelia.J. Exp. Biol. 78:149–161
Satow, Y., Kung, C. 1980a. Membrane currents of pawn mutants of the pwA group inParamecium tetraurelia.J. Exp. Biol. 84:57–71
Satow, Y., Kung, C. 1980b. Ca-induced K-outward current inParamecium tetraurelia.J. Exp. Biol. 88:293–303
Satow, Y., Kung, C. 1981. Possible reduction of surface charge by a mutation inParamecium tetraurelia.J. Membrane Biol. 59:179–190
Scarpa, A., Brinley, F.J., Tiffert, T., Dubyak, G.R. 1978. Metallochromic indicators of ionized calcium.Ann. N.Y. Acad. Sci. 307:86–112
Schein, S.J. 1976. Nonbehavioral selection for pawns, mutants ofParamecium aurelia with decreased excitability.Genetics 84:453–468
Silinsky, E.M. 1981. On the calcium receptor that mediates depolarization-secretion coupling at cholinergic motor nerve terminals.Br. J. Pharmacol. 73:413–429
Thiele, J., Honer-Schmid, O., Wahl, J., Kleefeld, G., Schultz, J.E. 1980. A new method for axenic mass cultivation ofParamecium tetraurelia.J. Protozool. 27:118–121
Thiele, J., Klumpp, S., Schultz, J.E., Bardele, C.F. 1982. Differential distribution of voltage-dependent calcium channels and guanylate cyclase in the excitable ciliary membrane fromParamecium tetraurelia.Eur. J. Cell Biol. 28:3–11
Thiele, J., Schultz, J.E. 1981. Ciliary membrane vesicles ofParamecium contain the voltage sensitive calcium channel.Proc. Natl. Acad. Sci. USA 78:3688–3691
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thiele, J., Otto, M.K., Deitmer, J.W. et al. Calcium channels of the excitable ciliary membrane fromParamecium: An initial biochemical characterization. J. Membrain Biol. 76, 253–260 (1983). https://doi.org/10.1007/BF01870367
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01870367