Summary
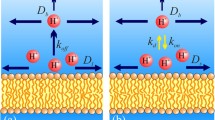
A simple carrier model describes adequately the transport of protons across lipid bilayer membranes by the weak acid S-13. We determined the adsorption coefficients of the anionic, A−, and neutral, HA, forms of the weak acid and the rate constants for the movement of A− and HA across the membrane by equilibrium dialysis, electrophoretic mobility, membrane potential, membrane conductance, and spectrophotometric measurements. These measurements agree with the results of voltage clamp and charge pulse kinetic experiments. We considered three mechanisms by which protons can cross the membranesolution interface. An anion adsorbed to the interface can be protonated by (i) a H+ ion in the aqueous phase (protolysis), (ii) a buffer molecule in the aqueous phase or (iii) water molecules (hydrolysis). We demonstrated that the first reaction cannot provide the required flux of protons: the rate at which H+ must combine with the adsorbed anions is greater than the rate at which diffusion-limited reactions occur in the bulk aqueous phase. We also ruled out the possibility that the buffer is the main source of protons: the rate at which buffers must combine with the adsorbed anions is greater than the diffusion-limited rate when we reduced the concentration of polyanionic buffer adjacent to the membrane-solution interface by using membranes with a negative surface charge. A simple analysis demonstrates that a hydrolysis reaction can account for the kinetic data. Experiments at acid pH demonstrate that the transfer of H+ from the membrane to the aqueous phase is limited by the rate at which OH combines with adsorbed HA and that the diffusion coefficient of OH− in the water adjacent to the bilayer has a value characteristic of bulk water. Our experimental results demonstrate that protons are capable of moving rapidly across the membrane-solution interface, which argues against some mechanisms of local chemiosmosis.
Similar content being viewed by others
References
Bakker, E.P., Arents, J.P., Hoebe, J.P.M., Terada, H. 1975. Surface potential and the interaction of weakly acidic uncouplers of oxidative phosphorylation with liposomes and mitochondria.Biochim. Biophys. Acta 387:491–506
Bangham, A.D., Hill, M.W., Miller, N.G.A. 1974. Preparation and use of liposomes as models of biological membranes.Methods Membr. Biol. 1:1–68
Barenholz, Y., Gibbes, D., Littman, B.J., Goll, J., Thompson, T.E. 1977. A simple method for the preparation of homogeneous phospholipid vesicles.Biochemistry 16:2806–2810
Bell, R.P. 1973. The Proton in Chemistry. Cornell University Press, Ithaca
Benz, R., McLaughlin, S. 1983. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanidep-trifluoromethoxyphenylhydrazone).Biophys. J. 41:381–398
Cohen, F.S., Eisenberg, M., McLaughlin, S. 1977. The kinetic mechanism of action of an uncoupler of oxidative phosphorylation.J. Membrane. Biol. 37:361–396
Delahay, P. 1954. New Instrumental Methods in Electrochemistry. pp. 87–95. Interscience. New York
Dijk, C. van, Levie, R. de 1985. An experimental comparison between the continuum and single jump descriptions of nonactin-mediated potassium transport through lipid membranes.Biophys. J. 48:125–136
Dilger, J., McLaughlin, S. 1979. Proton transport through membranes induced by weak acids: A study of two substituted benzimidazoles.J. Membrane Biol. 46:359–384
Dilger, J.P., Benz, R. 1985. Optical and electrical properties of thin monoolein lipid bilayers.J. Membrane Biol. 85:181–189
Eigen, M. 1964. Proton Transfer, Acid-Base Catalysis, and Enzymatic Hydrolysis: part I. Elementary Processes.Ange. Chem. Int. Ed. Engl. 3:1–72
Eigen, M., Kruse, W., Maass, G., De Maeyer, L. 1964. Rate constants of protolytic reactions in aqueous solution.Prog. React. Kinet. 2:287–318
Eisenberg, M., Gresalfi, T., Riccio, T., McLaughlin, S. 1979. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids.Biochemistry 18:5213–5223
Everitt, C.T., Redwood, W.R., Haydon, D.A. 1969. Problem of boundary layers in the exchange diffusion of water across bimolecular lipid membranes.J. Theor. Biol. 22:20–32
Finkelstein, A., Cass, A. 1968. Permeability and electrical properties of thin lipid membranes.J. Gen. Physiol. 52:145s-172s
Flewelling, R.F., Hubbell, W.L. 1986. Hydrophobic ion interactions with membranes: Thermodynamic analysis of tetraphenylphosphonium binding to vesicles.Biophys. J. 49:531–540
Flewelling, R.F., Hubbell, W.L. 1986. The membrane dipole potential in a self-consistent total membrane potential model.Biophys. J. 49:541–552
Gupte, S., Wu, E.-S., Hoechli, L., Hoechli, M., Jacobson, K., Sowers, A.EE., Hackenbrock, C.R. 1984. Relationship between lateral diffusion, collision frequency, and electron transfer of mitochondrial inner membrane oxidationreduction components.Proc. Natl. Acad. Sci. USA 81: 2606–2610
Gutknecht, J., Tosteson, D.C. 1973. Diffusion of weak acids across lipid bilayer membranes: Effects of chemical reactions in the unstirred layers.Science 182:1258–1261
Gutman, M. 1984. The pH jump: Probing of macromolecules and solutions by a laser-induced, ultrashort proton pulsetheory and applications in biochemistry.Methods Biochem. Anal. 30:1–103
Hall, J.E., Meed, C.A., Szabo, G. 1973. A barrier model for current flow in lipid bilayer membranes.J. Membrane Biol. 11:75–97
Hanstein, W.G., Hatefi, Y. 1974. Characterization and localization of mitochondrial uncoupler binding sites with an uncoupler capable of photoaffinity labeling.J. Biol. Chem. 249:1356–1362
Haydon, D.A., Hladky, S.B. 1974. Ion transport across thin lipid membranes. A Critical discussion of mechanisms in selected systems.Q. Rev. Biophys. 5:187–282
Helfferich, F. 1962. Ion Exchange. McGraw-Hill, New York
Hladky, S.B. 1974. The energy barriers to ion transport by nonactin across thin lipid bilayer membranes.Biochim. Biophys. Acta 352:71–85
Holz, R., Finkelstein, A. 1970. The water and nonelectrolyte permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B.J. Gen. Physiol. 56:125–145
Honig, B.H., Hubbell, W.L., Flewelling, R.F. 1986. Electrostatic interactions in membranes and proteins.Annu. Rev. Biophys. Biophys. Chem. 15:163–193
Israelachvili, J. N. 1985. Intermolecular and Surface Forces. Academic, New York
Kasianowicz, J., Benz, R., McLaughlin, S. 1984. The kinetic mechanism by which CCCP (carbonyl cyanidem-chlorophenylhydrazone) transports protons across membranes.J. Membrane Biol. 82:179–190
Katre, N.V., Wilson, D.F. 1977. Interaction of uncouplers with the mitochondrial membrane: A high-affinity binding site.Arch. Biochem.Biophys. 184:578–585
Katre, N.V., Wilson, D.F. 1978. Interaction of uncouplers with the mitochondrial membrane: Identification of the high affinity binding site.Arch. Biochem. Biophys. 191:647–656
Kell, D.B. 1979. On the functional proton current pathway of electron transport phosphorylation. An electrodic view.Biochim. Biophys. Acta 549:55–99
Lauger, P., Neumcke, B. 1973. Theoretical analysis of ionconductance in lipid bilayer membranes.In: Membranes, a Series of Advances G. Eisenman, editor. Vol. 2, pp. 1–59. Dekker, New York
LeBlanc, O.H., Jr. 1971. The effect of uncouplers of oxidative phosphorylation on lipid bilayer membranes. Carbonylcyanidem-chlorophenylhydrazone.J. Membrane Biol. 4:227–251
Lowry, R.R., Tinsley, I.J. 1974. A simple sensitive method for lipid phosphorus.Lipids 9:491–492
McLaughlin, S. 1977. Electrostatic potentials at membranesolution interfaces.Curr. Top. Membr. Transp. 9:71–144
McLaughlin, S.G.A., Dilger, J.P. 1980. Transport of protons across membranes by weak acids.Physiol. Rev. 60:825–863
McLaughlin, S., Eisenberg, M. 1975. Antibiotics and membrane biology.Annu. Rev. Biophys. Bioeng. 4:335–366
Mitchell, P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism.Nature (London) 191:144–148
Nachliel, E., Gutman, M. 1984. Kinetic analysis of proton transfer between reactants adsorbed to the same micelle. The effect of proximity on the rate constants.Eur. J. Biochem. 143:83–88
Neumcke, B., Bamberg, E. 1975. The action of uncouplers on lipid bilayer membranes.In: Membranes. Vol. 3. G. Eisenman, editor. Dekker, New York
Neumcke, B., Lauger, P. 1969. Nonlinear electrical effects in lipid bilayer membranes: II. Integration of the generalized Nernst-Planck equations.Biophys. J. 9:1160–1170
O'Shaughnessy, K., Hladky, S.B. 1983. Transient currents carried by the uncoupler, carbonyl cyanidem-chlorophenylhydrazone.Biochim. Biophys. Acta 724:381–387
Parsegian, A. 1969. Energy of an ion crossing a low dielectric membrane. Solutions to four relevant electrostatics problems.Nature (London) 221:844–846
Parsegian, A., Fuller, N., Rand, P. 1979. Measured work of deformation and repulsion of lecithin bilayers.Proc. Natl. Acad. Sci. USA. 76:2750–2754
Prats, M., Teissie, J., Tocanne, J.-F. 1986. Lateral proton conduction at lipid water interfaces and its implications for the chemiosmotic-coupling hypothesis.Nature (London) 322:756–758
Smejtek, P., Hsu, K., Perman, W.H. 1976. Electrical conductivity in lipid bilayer membranes induced by pentachlorophenol.Biophys. J. 16:319–336
Szabo, G., Eisenman, G., McLaughlin, S.G.A., Krasne, S. 1972. Ionic probes of membrane structures.Ann. N.Y. Acad. Sci. 195:273–290
Teissie, J., Prats, M., Soucaille, P., Tocanne, J.F. 1985. Evidence for conduction of protons along the interface between water and a polar lipid monolayer.Proc. Natl. Acad. Sci. USA 82:3217–3221
Terada, H. 1981. The interaction of highly active uncouplers of oxidative phosphorylation with mitochondria.Biochim. Biophys. Acta 639:225–242
Terada, H., Van Dam, K. 1975. On the stoichiometry between uncouplers of oxidative phosphorylation and respiratory chains: The catalytic action of SF-6847 (3,5-di-tert-butyl-4-hydroxybenzylidenemalonitrile).Biochim. Biophys. Acta 387:507–518
Tsui, F.C., Ojcius, D.M., Hubbell, W.L. 1985. The intrinsic pKa values for phosphatidylserine and phosphatidylethanolamine in phosphatidylcholine host bilayers.Biophys. J. 49:459–468
Vetter, K.J. 1967. Electrochemical Kinetics: Theoretical Aspects. Academic, New York
Westerhoff, H.V., Chen, Y.-D. 1985. Stochastic free energy transduction: The role of independent, small coupling units.Biochim. Biophys. Acta 768:257–292
Westerhoff, H.V., Melandri, B.A., Venturoli, G., Azzione, G.F., Kell, D.B. 1984. A minimal hypothesis for membranelinked free-energy transduction: The role of independent, small coupling units.Biochim. Biophys. Acta 768:257–292
Wilson, D.F., Ting, H.P., Koppleman, M.S. 1971. Mechanism of action of uncouplers of oxidative phosphorylation.Biochemistry 10:2897–2902
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kasianowicz, J., Benz, R. & McLaughlin, S. How do protons cross the membrane-solution interface? Kinetic studies on bilayer membranes exposed to the protonophore S-13 (5-chloro-3-tert-butyl-2′-chloro-4′ nitrosalicylanilide). J. Membrain Biol. 95, 73–89 (1987). https://doi.org/10.1007/BF01869632
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01869632