Summary
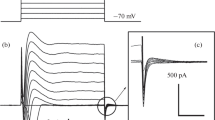
The membrane ofParamecium generates a Ca-dependent Na current upon depolarization. There is, however, also a Na current upon hyperpolarization in this membrane. The second Na current was analyzed under voltage clamp and found to have properties identical to those of the first. Both currents could be carried by Na and Li ions and not by K, Cs or choline ion. They were eliminated by either EGTA injection into the cell or Ca removal from the bath. Both currents were eliminated by a single-gene mutation,fast-2, that had no effect on Ca currents. These findings strongly suggest that these two currents are through the same Ca-dependent Na conductance. A hyperpolarization-induced Ca current was also identified, which served to activate the second Na current. These observations support a model that theParamecium membrane has two Ca channels with different voltage dependencies and only one Na channel, which is elicited by a rise of the itternal free Ca2+ concentration. The function of the Ca-dependent Na conductance is discussed.
Similar content being viewed by others
References
Aldenhoff, J.B., Hofmeier, G., Lux, H.D., Swandulla, D. 1983. Stimulation of a sodium influx by cAMP inHelix neurons.Brain Res. 276:289–296.
Baker, P.F., Hodgkin, A.L., Ridgeway, E.B. 1971. Depolarization and calcium entry in squid giant axons.J. Physiol. (London) 218:709–755
Brehm, P., Eckert, R., Tillotson, D. 1980. Calcium-mediated inactivation of calcium current inParamecium.J. Physiol. (London) 306:193–203
Capovilla, M., Caretta, A., Cervetto, L., Torre, V. 1983. Ionoic movements through light-sensitive channels of toad rods.J. Physiol. (London) 343:295–310
Colquhoun, D., Neher, E., Reuter, H., Stevens, C.F. 1981. Inward current channels activated by intracellular Ca in cultured cardiac cells.Nature (London) 294:752–754
Conner, J.A., Hockberger, P. 1984. A novel membrane sodium current induced by injection of cyclic nucleotides into gastropod neurones.J. Physiol. (London) 354:139–162
Conner, J.A., Stevens, C.F. 1971. Prediction of repetitive firing behavior from voltage clamp data on an isolated neurone soma.J. Physiol. (London) 213:31–53
Connolly, J.G., Kerkut, G.A. 1983. Ion regulation and membrane potential inTetrahymena andParamecium.Comp. Biochem. Physiol. 76A:1–16
Fesenko, E.E., Kolesnikov, S., Lyubarsky, A.L. 1985. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment.Nature (London) 313:310–313
Hansma, H.G. 1979. Sodium uptake and membrane excitation inParamecium.J. Cell Biol. 81:374–381
Hermann, A., Gorman, A.L.F. 1981. Effects of tetraethylammonium on potassium currents in a molluscan neurone.J. Gen. Physiol. 78:87–110
Hinrichsen, R.D., Saimi, Y. 1984. A mutation that alters properties of the calcium channel inParamecium tetraurelia.J. Physiol. (London) 351:397–410
Hinrichsen, R.D., Saimi, Y., Kung, C. 1984. Mutants with altered Ca2+-channel properties inParamecium tetraurelia: Isolation, characterization and genetic analysis.Genetics 108:545–558
Hodgkin, A.L., McNaughton, P.A., Nunn, B.J. 1985. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods.J. Physiol. (London) 358:447–468
Kung, C. 1971. Genic mutants with altered system of excitation inParamecium aurelia: I. Phenotypes of the behavioral mutants.Zeit. verg. Physiol. 71:142–164
Kung, C., Chang, S.-Y., Satow, Y., Van Houten, J., Hansma, H.G. 1975. Genetic dissection of behavior inParamecium.Science 188:898–904
Kung, C., Saimi, Y. 1982. The physiological basis of taxes inParamecium.Annu. Rev. Physiol. 44:519–534
Machemer, H., Ogura, A. 1979. Ionic conductances of membranes in ciliated and deciliatedParamecium.J. Physiol. (London) 296:49–60
Maruyama, Y., Petersen, O.H. 1982. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini.Nature (London) 299:159–161
Mathews, H.R., Torre, V., Lamb, T.D. 1985. Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods.Nature (London) 313:582–585
Meves, H., Vogel, W. 1973. Calcium inward currents in internally perfused giant axons.J. Physiol. (London) 235:225–265
Moczydlowski, E., Latorre, R. 1983. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers: Evidence for two voltage-dependent Ca2+ binding reactions.J. Gen. Physiol. 83:511–542
Saimi, Y., Hinrichsen, R.D., Forte, H., Kung, C. 1983. Mutant analysis shows that the Ca2+-induced K+ current shuts off one type of excitation inParamecium.Proc. Natl. Acad. Sci. USA 80:5112–5116
Saimi, Y., Kung, C. 1980. A Ca-induced Na+ current inParamecium.J. Exp. Biol. 88:305–325
Satow, Y., Kung, C. 1974. Genetic discussion of active electrogenesis inParamecium aurelia.Nature (London) 247:69–71
Satow, Y., Kung, C. 1976. A mutant ofParamecium with increased relative resting potassium permeability.J. Neurobiol. 7:325–338
Sonneborn, T.M. 1975.Paramecium aurelia:In: Handbook of Genetics. Vol.II, pp. 469–594. R.C. King, editor. Plenum, New York
Swandulla, D., Lux, H.D. 1984. Changes in ionic conductances induced by cAMP inHelix neurons.Brain Res. 305:115–122
Yau, K.-W., Nakatani, K. 1984. Cation selectivity of light-sensitive conductance in retinal rods.Nature (London) 309:352–354
Yellen, G. 1982. Single Ca2+-activated nonselective cation channels in neuroroblastoma.Nature (London) 296:357–359
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saimi, Y. Calcium-dependent sodium currents inParamecium: Mutational manipulations and effects of hyper- and depolarization. J. Membrain Biol. 92, 227–236 (1986). https://doi.org/10.1007/BF01869391
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01869391