Summary
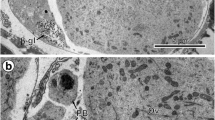
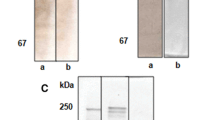
An assay has been developed for quantitating the reassociation of cortical secretory vesicles (CVs) with fragments of sea urchin egg plasma membrane attached to glass slides (PM lawns). Binding ofS. pupuratus CVs to homologous PM lawns increased with time and CV concentration. The observation that CV binding was blocked by chymotrypsin digestion of the PM fragments suggested that a PM protein(s) is required for reassociation. The possibility that the extent of CV lysis that occurred during CV preparation (15.4±3.8% as assessed by ovoperoxidase assay) influenced reassociation was investigated by determining the effect of CV content proteins (isolated as fertilization product) on binding. Various concentrations of fertilization product (up to equivalent amounts of fertilization product and CV protein) had no effect on CV binding. The specificity of binding was investigated by assessing the ability of CVs to bind to PM lawns prepared from human red blood cells, and by determining the ability of heterologous vesicles to bind to egg PM fragments. PM lawns from HRBCs did not support CV binding; however, PM lawns prepared from the eggs of several species of sea urchin did bindS. pupuratus CVs. Vesicles from a partially purified preparation of yolk platelets bound to egg PM lawns with low efficiency (1/7 that of CVs), but immunofluorescence analysis with an anti-hyalin monoclonal antibody demonstrated that 74±9% of the bound vesicles were CVs that contaminated the yolk platelet preparation. Dioleoylphosphatidyl choline liposomes were also unable to bind to egg PM lawns. These results are consistent with hypothesis that CV binding to egg PM lawns is a specific, protein-mediated event.
Similar content being viewed by others
References
Anderson, E. 1970. A cytological study of the centrifuged whole, half, and quarter eggs of the sea urchinArbacia punctulata.J. Cell Biol. 47:711–733
Begg, D.A., Rebhun, L.L. 1979. pH regulates the polymerization of the actin in the sea urchin egg cortex.J. Cell. Biol. 83:242–248
Bonder, E.M., Fishkind, D.J., Cotran, N.M., Begg, D.A. 1989 Cortical actin-membrane cytoskeleton of unfertilized sea urchin eggs: Analysis of the spatial organization and relationship of filamentous actin, nonfilamentous actin, and egg spectrin.Dev. Biol. 134:327–241
Bradford, M.M. 1976. A rapid and sensitive model for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal. Biochem. 72:248–254
Chandler, D. 1984. Exocytosis in vitro: Ultrastructure of the isolated sea urchin cortex as seen in platinum replicas:J. Ultrastruct. Res. 89:198–211
Clapper, D.L., Lee, H.C. 1985. Inositol trisphosphate induces calcium release from nonmitochondrial stores in sea urchin egg homogenates.J. Biol. Chem. 260:13947–13954
Crabb, J.H. 1986. In vitro reconstitution of exocytosis from sea urchin egg plasma membrane and isolated secretory vesicles. Ph.D. Thesis, Dartmouth College, Hanover (NH)
Crabb, J.H., Jackson, R.C. 1985. In vitro reconstitution of exocytosis from plasma membrane and isolated secretory vesicles.J. Cell. Biol. 101:2263–2273
Crabb, J.H., Jackson, R.C. 1986. Polycation inhibition of exocytosis from sea urchin egg cortex.J. Membrane Biol. 91:85–96
Crabb, J.H., Modern, P.A., Jackson, R.C. 1987. In vitro reconstitution of exocytosis from sea urchin egg plasma membrane and isolated cortical vesicles.Biosci. Rep. 7:399–409
Detering, N.K., Decker, G.L., Schmell, E.D., Lennarz, W.J. 1977. Isolation and characterization of plasma membrane-associated cortical granules from sea urchin eggs.J. Cell. Biol. 75:899–914
Diets, T., Farrance, M., Kay, E.S., Medill, L., Turner, E.E., Weidman, P.J., Shapiro, B.M. 1984. Purification and properties of ovoperoxidase, the enzyme responsible for hardening the fertilization membrane of the sea urchin egg.J. Biol. Chem. 259:13525–13533
Goding, J.W. 1976. Conjugation of antibodies with fluorochromes: Modifications to the standard methods.J. Immunol. Methods 13:215–226
Henson, J.H., Beaulieu, S.M., Begg, D.A., Fishkind, D.J., Bonder, E.M., Lebeche, D., Kanimer, B. 1988. Localization of a calsequestrin-like protein in the ER of sea urchin eggs.J. Cell. Biol. 107:172a
Henson, J.H., Begg, D.A. 1988. Filamentous actin organization in the unfertilized sea urchin egg cortex.Dev. Biol. 127:338–348
Hylander, B.L., Summers, R.G. 1981. The effect of local anesthetic and ammonia on cortical granule-plasma membrane attachment in the sea urchin egg.Dev. Biol. 86:1–11
Ii, I., Deguchi, K., Kawashima, S., Endo, S., Ueta, N. 1978. Water soluble lipoproteins from yolk granules in sea urchin eggs. I. Isolation and general properties.J. Biochem. (Tokyo) 84:737–749.
Jackson, R.C., Ward, K.K., Haggerty, J.G. 1985. The effect of proteolytic digestion on the release of cortical vesicle contents from the cell surface complex of the sea urchin egg.J. Cell Biol. 101:6–11 (Erratum:101:1167)
Jacobson, B.S., Branton, D. 1977. Plasma membrane: Rapid isolation and exposure of the cytoplasmic surface by the use of positively charged beads.Science 195:302–304
Kay, E.S., Shapiro, B.M. 1985. The formation of the fertilization membrane of the sea urchin egg.In: Biology of Fertilization. C.B. Metz and A. Monroy, editors. Vol. 3, pp. 45–80. Academic, Orlando (FL)
Kopf, G.S., Moy, G.W., Vacquier, V.D. 1982. Isolation and characterization of sea urchin egg cortical granules.J. Cell Biol. 95:924–932
Mabuchi, I., Hosoya, H., Sakai, H. 1980. Actin in the cortical layer of the sea urchin egg. Changes in its content during and after fertilization.Biomed. Res. 1:417–426
Moser, R. 1939. Studies on a cortical layer response to stimulating agents in theArbacia egg.J. Exp. Zool. 80:423–445
Oberdorf, J.A., Head, J.F., Kaminer, B. 1986. Calcium uptake and release by isolated cortices and microsomes from the unfertilized egg of the sea urchinStrongylocentrotus droebachiensis.J. Cell Biol. 102:2205–2210
Otto, J.J., Kane, R.E., Bryan, J. 1980. Redistribution of actin and fascin in sea urchin eggs after fertilization.Cell Motil. 1:31–41
Rothschild, L., Swann, M.M. 1949. The fertilization reaction in the sea urchin egg: A propogated response to sperm attachment.J. Exp. Biol. 26:164–176
Sardet, C. 1984. The ultrastructure of the sea urchin egg cortex isolated before and after fertilization.J. Cell. Biol. 105:196–210
Schatten, H., Cheney, R., Balczon, R., Willard, M., Cline, C., Simerly, C., Shatten, G. 1986. Localization of fodrin during fertilization and early development of sea urchins and mice.Dev. Biol. 118:457–477
Spudich, A., Spudich, J.A. 1979. Actin in Triton-treated cortical preparations of unfertilized and fertilized sea urchin eggs.J. Cell Biol. 83:212–226
Spudich, A., Wrenn, J.T., Wessells, N.K. 1988. Unfertilized sea urchin eggs contain a discrete cortical shell of actin that is subdivided into organizational states.Cell Motil. Cytoskel. 9:85–96
Swann, K., Whitaker, M.J. 1986. The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs.J. Cell Biol. 103:2333–2342
Turner, P.R., Jaffe, L.A., Fein, A. 1986. Regulation of cortical vesicle exocytosis in sea urchin eggs by inositol 1,4,5-trisphosphate and GTP-binding protein.J. Cell. Biol. 102:70–76
Ursitti, J.A., Strong, J., Pumplin, D.W., Bloch, R.J. 1988. Ultrastructural studies of the intact human erythrocyte cytoskeleton.J. Cell. Biol. 107:469a
Vacquier, V.D. 1975. The isolation of intact cortical granules from sea urchin eggs. Calcium ions trigger granule discharge.Dev. Biol. 43:62–74
Vacquier, V.D., Moy, G.W. 1980. The cytolytic isolation of the cortex of the sea urchin egg.Dev. Biol. 77:178–190
Vater, C.A., Jackson, R.C. 1989a. Identification of a major polypeptide component of the sea urchin fertilization envelope.Dev. Biol. 132:113–129
Vater, C.A., Jackson, R.C. 1989b. Purification and characterization of a cortical secretory vesicle membrane fraction.Dev. Biol. 135:111–123
Weidman, P.J., Kay, E.S. 1986. Egg and embryonic extracellular coats: Isolation and purification.Methods Cell Biol. 27:111–138
Whalley, T., Whitaker, M. 1988. Exocytosis reconstituted from the sea urchin egg is unaffected by calcium pretreatment of granules and plasma membrane.Biosci. Rep. 8:335–343
Whitaker, M.J., Baker, P.F. 1983. Calcium-dependent exocytosis in an in vitro secretory granule plasma membrane preparation from sea urchin eggs and the effects of some inhibitors of cytoskeletal function.Proc. R. Soc. London B 218:397–413
Whitaker, M.J., Steinhardt, R.A. 1985. Ionic signaling in the sea urchin egg at fertilization.In: Biology of Fertilization. C.B. Metz and A. Monroy, Editors. Vol. 3, pp. 168–221. Academic, Orlando (FL)
Zimmerberg, J., Sardet, C., Epel, D. 1985. Exocytosis of sea urchin cortical vesicles in vitro is retarded by hypersomatic sucrose: Kinetics of fusion monitored by quantitative light scattering microscopy.J. Cell Biol. 101:2398–2410
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jackson, R.C., Modern, P.A. Reassociation of cortical secretory vesicles with sea urchin egg plasma membrane: Assessment of binding specificity. J. Membrain Biol. 115, 83–93 (1990). https://doi.org/10.1007/BF01869108
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01869108