Summary
Ion-carrier complexes and organic ions of similar size and shape have mobilities in lipid bilayer membranes which span several orders of magnitude. In this communication, an examination is made of the hypothesis that the basis for this unusually wide range of ionic mobilities is the potential energy barrier arising from image forces which selectively act on ions according to their polarizability. Using Poisson's equation to evaluate the electrostatic interaction between an ion and its surroundings, the potential energy barrier to ion transport due to image effects is computed, with the result that the potential energy barrier height depends strongly on ionic polarizability.
Theoretical membrane potential energy profile calculations are used in conjunction with the Nernst-Planck electrodiffusion equation to analyze the available mobility data for several ion-carrier complexes and lipid-soluble ions in lipid bilayer membranes. The variation among the mobilities of different ions is shown to be in agreement with theoretical predictions based on ionic polarizability and size. Furthermore, the important influence exerted by image forces on ion transport in lipid bilayer membranes compared to the frictional effect of membrane viscosity is established by contrasting available data on the activation energy of ionic conductivity with that for membrane fluidity.
Similar content being viewed by others
Abbreviations
- A :
-
spherical conductor radius
- c :
-
ionic concentration in the membrane
- c w :
-
ionic concentration in the aqueous solution
- E :
-
electric field strength/(RT/ℱL)
- e :
-
elementary charge
- F :
-
image force
- ΔH η :
-
activation energy for microviscosity
- ΔH k :
-
activation energy for electrodiffusion
- J :
-
current flux
- k :
-
electrodiffusion rate constant for lim ψ→0 defined byJ=ckψLℱ
- k s :
-
modified electrodiffusion rate constant
- L :
-
membrane width
- P :
-
arbitrary image charge from one of Eqs. (14)–(17)
- Q :
-
ionic charge
- R :
-
gas law constant
- r :
-
ionic radius
- S :
-
sum of image charges within spherical conductor
- T :
-
absolute temperature
- u :
-
Stokes-Einstein mobility (footnote 1)
- α:
-
polarizability
- β:
-
(εI−εII)/(εI+εII)
- γ:
-
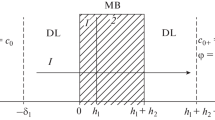
see Fig. 2
- δ:
-
see Fig. 2
- ε:
-
dielectric constant
- ε0 :
-
permittivity of free space
- ζ:
-
radial space coordinate/L
- η:
-
microviscosity relative to di(18∶1)-PC
- θ:
-
see Fig. 2
- Θ:
-
ionic charge/e
- Λ:
-
constant defined in Eq.(44)
- λ:
-
A/L
- μ:
-
Poisson's equation constant
- ξ:
-
axial space coordinate/L
- P:
-
image charge/e
- ϱ:
-
space charge density/(e/L 3)
- Φ:
-
effective potential energy barrier
- φ:
-
potential energy/RT
- χ:
-
separation distance between an arbitrary charge and a charged spherical conductor
- ψ:
-
electric potential/(RT/ℱ)
- ∇2 :
-
Laplacian
- ℱ:
-
Faraday constant
- D :
-
Stokes-Einstein diffusivity
- ℓ:
-
L/L(di(18∶1)-PC)
- C :
-
chemical
- E :
-
electrostatic
- M, N :
-
image charge indices
- Q :
-
refers to ion (source charge)
- R :
-
refers to location of ion binding site within the membrane
- I, II, III:
-
dielectric regions
- *:
-
denotes maximum potential energy, ϕ * E
- TPhB− :
-
tetraphenylboride anion
- DPA− :
-
dipicrylamine anion
- CCCP− :
-
carbonylcyanidem-chlorophenylhydrazone anion
- egg PC:
-
phosphatidyl choline from egg yolk
- di(N∶1)-PC:
-
diacylphosphatidyl choline of an N-carbon mono-unsaturated fatty acid
- GMO:
-
glyceryl monooleate
- GMER:
-
glyceryl monoerucin
- CHL:
-
cholesterol
- PI:
-
phosphatidyl inositol
- PS:
-
phosphatidyl serine
- DPPC:
-
dipalmitoyl phosphatidyl choline
- BPE:
-
bacterial phosphatidyl ethanolamine
- PE:
-
phosphatidyl ethanolamine
References
Andersen, O.S., Fuchs, M. 1975. Potential energy barriers to ion transport within lipid bilayers: Studies with tetraphylborate.Biophys. J. 15:795
Anderson, J.E., Jackson, H.W. 1974. Membrane-water partition coefficients of ions. Calculated effects of membrane thickness.J. Phys. Chem. 78:2259
Barker, R.E., Jr., Thomas, C.R. 1964. Glass transition and ionic conductivity in cellulose acetate.J. Appl. Phys. 35:87
Barrer, R.M., Rees, L.V.C. 1960. Energies of activation for self-diffusion of alkali metal ions in analcite.Nature 187:768
Benz, R., Stark, G. 1975. Kinetics of macrotetralide-induced ion transport across lipid bilayer membranes.Biochim. Biophys. Acta 382:27
Benz, R., Stark, G., Janko, K., Läuger, P. 1973. Valinomycin-mediated ion transport through neutral lipid membranes: Influence of hydrocarbon chain length and temperature.J. Membrane Biol. 14:339
Bondi, A. 1968. Physical Properties of Molecular Crystals, Liquids and Glasses. pp. 453–468. Wiley, New York
Böttcher, C.J.F. 1973. Theory of Electric Polarization Vol. I, p. 86. Elsevier Publishing Co., New York
Ciani, S., Laprade, R., Eisenman, G. Szabo, G. 1973. Theory for carrier-mediated zerocurrent conductance of bilayers extended to allow for nonequilibrium of interfacial reactions, spatially dependent mobilities and barrier shape.J. Membrane Biol. 11:255
Cogan, U., Shinitzky, M., Weber, G., Nishida, T. 1973. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes.Biochemistry 12:521
Coster, H.G.L., Smith, J.R. 1974. The molecular organization of bimolecular lipid membranes. A study of the low frequency Maxwell-Wagner impedance.Biochim. Biophys. Acta 373:151
Fettiplace, R., Andrews, D.M., Haydon, D.A. 1971. The thickness, composition and structure of some lipid bilayers and natural membranes.J. Membrane Biol. 5:277
Fuoss, R.M., Hirsch, E. 1960. Single ion conductances in non-aqueous solvents.J. Amer. Chem. Soc. 82:1013
Galla, H.-J., Sackmann, E. 1974. Lateral diffusion in the hydrophobic region of membranes: Use of pyrene excimers as optical probes.Biochim. Biophys. Acta 339:103
Gambale, F., Gliozzi, A., Robello, M. 1973. Determination of rate constants in carrier-mediated diffusion through lipid bilayers.Biochim. Biophys. Acta 330:325
Gilkerson, W.R., Srivastava, K.K. 1961. The dielectric properties of tetra-n-butylammonium picrate, bromide and tetraphenylboride in some polar solvents at 25°C.J. Phys. Chem. 65:272
Gilkerson, W.R., Stewart, J.C. 1961. Polarizabilities and molar volumes of a number of salts in several solvents at 25°C.J. Phys. Chem. 65:1465
Hall, J.E., Mead, C.A., Szabo, G. 1973. A barrier model for current flow in lipid bilayer membranes.J. Membrane Biol. 11:75
Haydon, D.A., Hladky, S.B. 1972. Ion transport across thin lipid membranes: A critical discussion of mechanisms in selected systems.Q. Rev. Biophys. 5:187
Hladky, S.B. 1974. The energy barriers to ion transport by nonactin across thin lipid membranes.Biochim. Biophys. Acta 352:71
Hladky, S.B., Gordon, C.G.M., Haydon, D.A. 1974. Molecular mechanisms of ion transport in lipid membranes.Annu. Rev. Phys. Chem. 25:11
Jacobsen, K., Wobschall, D. 1974. Rotation of fluorescent probes localized within lipid bilayer membranes.Chem. Phys. Lipids 12:117
Ketterer, B., Neumcke, B., Läuger, P. 1971. Transport mechanism of hydrophobic ions through lipid bilayer membranes.J. Membrane Biol. 5:225
Krasne, S., Eisenman, G. 1973. The molecular basis of ion selectivity.In: Membranes. A Series of Advances. G. Eisenman, editor. p. 277. Marcel Dekker, Inc., New York
Krasne, S., Eisenman, G., Szabo, G. 1971. Freezing and melting of lipid bilayers and the mode of action of monactin, valinomycin and gramicidin.Science 174:412
LeBlanc, O.H., Jr. 1969. Tetraphenylborate conductance through lipid bilayer membranes.Biochim. Biophys. Acta 193:350
LeBlanc, O.H., Jr. 1971. Effect of uncouplers of oxidative phosphorylation on lipid bilayer membranes: Carbonylcynanidem-chlorophenylhydrazone.J. Membrane Biol. 4:227
Neumcke, B., Läuger, P. 1969. Non-linear electrical effects in lipid bilayer membranes. II. Integration of the generalized Nernst-Planck equations.Biophys. J. 9:1160
Parsegian, A. 1969. Energy of an ion crossing a low dielectric membrane: Solutions to four relevant electrostatic problems.Nature 221:844
Rosenberg, B., Bhowmik, B.B. 1969. Donor-acceptor complexes and the semiconductivity of lipids.Chem. Phys. Lipids 3:109
Ryan, T.H., Koryta, J., Hofananova-Matjekova, A., Brezina, M. 1974. Polarography of alkali metal ion complexes of macrotetralides: Complex ion size and stability.Anal. Lett. 7:335
Shinitzky, M., Barenholz, Y. 1974. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate.J. Biol. Chem. 249:2652
Simon, W., Morf, W.E., Meier, P.Ch. 1973. Specificity for alkali and alkaline earth cations of synthetic and natural organic complexing agents in membranes.Struct. Bonding (Berlin) 16:113
Smythe, W.R. 1968. Static and Dynamic Electricity. p. 22. McGraw-Hill, New York
Stark, G., Benz, R., Pohl, G.W., Janko, K. 1972. Valinomycin as a probe for the study of structural changes of black lipid membranes.Biochim. Biophys. Acta 266:603
Stark, G., Ketterer, B., Benz, R., Läuger, P. 1971. The rate constants of valinomycin-mediated ion transport through thin lipid membranes.Biophys. J. 11:981
Szabo, G. 1973. Location of cations complexed by neutral carriers in lipid bilayer membranes.Biophys. J. 15:306a
Tredgold, R.H. 1973. Hydration energy and the transport of ions through membranes.Biochim. Biophys. Acta 323:143
Vanderkooi, J.M., Callis, J.B. 1974. Pyrene. A probe of lateral diffusion in the hydrophobic region of membranes.Biochemistry 13:4000
Vanderkooi, J., Fischkoff, S., Chance, B., Cooper, R.A. 1974. Fluorescent probe analysis of the lipid architecture of natural and experimental cholesterol-rich membranes.Biochemistry 13:1589
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bradshaw, R.W., Robertson, C.R. Effect of ionic polarizability on electrodiffusion in lipid bilayer membranes. J. Membrain Biol. 25, 93–114 (1975). https://doi.org/10.1007/BF01868570
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01868570