Summary
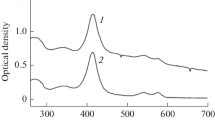
Human red blood cell membranes were solubilized with sodium dodecylsulfate and incubated with various concentrations of14C-glucose and14C-sorbose. After gel filtration on Sephadex G-100, which separated lipoproteins of differing lipid content, it was observed that the radioactivity of the bound glucose coincided with the protein peak. Radioactivity of bound sorbose was found mainly before and after the protein peak. This distribution of bound sugars was confirmed by double labeling experiments in which3H-glucose and14C-sorbose were applied simultaneously. Infrared spectroscopy revealed differences between the membranes loaded with sorbose and glucose. Particularly, the band in the C−O−C and P=O region at 1,225 cm−1 was intensified in the sorbose-loaded membranes. Compared to serum albumin, the erythrocyte membranes were found to bind 4 times as much14C-glucose per mg of protein. It is concluded from the results obtained by gel filtration that glucose and sorbose preferentially bind at different sites of the erythrocyte membrane. The results obtained by infrared spectroscopy correspond with this conclusion.
Similar content being viewed by others
References
Bakerman, S., Wasemiller, G. 1967. Studies of the structural units of human erythrocyte membrane. I. Separation, isolation and partial characterization.Biochemistry 6:1100.
Blumenfeld, O. O. 1968. The proteins of the erythrocyte membrane obtained by solubilization with aqueous pyridine solution.Biochem. Biophys. Res. Commun. 30:200.
Chapman, D., Kamat, V. B., Levene, R. J. 1968. Infrared spectra and the chain organization of erythrocyte membranes.Science 160:314.
Dodge, J. T., Mitchell, C., Hanahan, D. J. 1963. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes.Arch. Biochem. Biophys. 100:119.
Hoogeveen, J. Th., Juliano, R., Coleman, J., Rothstein, A. 1970. Water-soluble proteins of the human red cell membrane.J. Membrane Biol. 3:156.
Hummel, J. P., Dreyer, W. J. 1962. Measurement of protein-binding phenomena by gel filtration.Biochim. Biophys. Acta 63:530.
Lacko, L. 1967. Specificity of sugar carriers in erythrocytes.Nature 213:523.
Lacko, L., Burger, M. 1961. Common carrier system for sugar transport in human red cells.Nature 191:881.
LeFevre, P. G. 1954. The evidence for active transport of monosaccharides across the red cell membrane.Symp. Soc. Exp. Biol. No. VIII, Active Transport and Secretion. p. 118.
Lenard, J. 1970. Protein and glycolipid components of human erythrocyte membranes.Biochemistry 9:1129.
Levine, M., Oxender, D. L., Stein, W. D. 1965. The substrate-facilitated transport of the glucose carrier across the human erythrocyte membrane.Biochim. Biophys. Acta 109:151.
Levine, M., Stein, W. D. 1966. The kinetic parameters of the monosaccharide transfer system of the human erythrocyte.Biochim. Biophys. Acta 127:179.
Lieb, W. R., Stein, W. D. 1970. Quantitative predictions of a noncarrier model for glucose transport across the human red cell membrane.Biophys. J. 10:585.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J. 1951. Protein measurement with the Folin phenol reagent.J. Biol. Chem. 193:265.
Maddy, A. H. 1966. The properties of the protein of the plasma membrane of ox erythrocytes.Biochim. Biophys. Acta 117:193.
Marchesi, S. L., Steers, E., Marchesi, V. T., Tillack, T. W. 1970. Physical and chemical properties of a protein isolated from red cell membranes.Biochemistry 9:50.
Marchesi, V. T., Steers, E., Jr. 1968. Selective solubilization of a protein component of the red cell membrane.Science 159:203.
Miyazawa, T., Blout, E. R. 1961. The infrared spectra of polypeptides in various conformations: Amide I and II bands.J. Amer. Chem. Soc. 83:712.
Rosenberg, S. A., Guidotti, G. 1969. Fractionation of the protein components of human erythrocyte membranes.J. Biol. Chem. 244:5118.
Sen, A. K., Widdas, W. F. 1962. Determination of the temperature and pH dependence of glucose transfer across the human erythrocyte membrane measured by glucose exit.J. Physiol. 160:392.
Stein, W. D. 1969. Transport proteins. Intra-protein interactions across a fluid membrane as a model for biological transport.J. Gen. Physiol. 54:81S.
Tipson, R. S., Isbell, H. S. 1962. Infrared absorption spectra in the study of mutarotational equilibria of monosaccharides.J. Phys. Nat. Bur. Stand. 66A:31.
Wallach, D. F. H. 1969. Membrane lipids and the conformations of membrane proteins.J. Gen. Physiol. 54:3S.
Wallach, D. F. H., Zahler, P. H. 1966. Protein conformations in cellular membranes.Proc. Nat. Acad. Sci. 56:1552.
Wilbrandt, W. 1960. The sugar transport across the red cell membrane.In: Symposium on Membrane Transport and Metabolism. A. Kleinzeller and A. Kotyk, editors. p. 205. Publishing House, Czechoslovak Academy of Sciences, Prague.
Wood, G. C., Cooper, P. F. 1970. The application of gel filtration to the study of protein-binding of small molecules.Chromatogr. Rev. 12:88.
Zimmer, G., Lacko, L. 1971. Structural change of human red cell membranes in the glucose-preloaded state.F. E. B. S. 12:333.
Zöllner, N., Kirsch, K. 1962. Über die quantitative Bestimmung von Lipoiden (Mikromethode) mittels der vielen natürlichen Lipoiden (allen bekannten Plasmalipoiden) gemeinsamen Sulphophosphovanillin-Reaktion.Z. Ges. Exp. Med. 135:545.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zimmer, G., Lacko, L. & Günther, H. Different binding sites for glucose and sorbose at the erythrocyte membrane, studied by gel filtration and infrared spectroscopy. J. Membrain Biol. 9, 305–318 (1972). https://doi.org/10.1007/BF01868059
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01868059