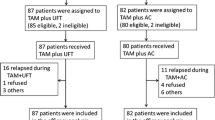
Summary
Eighty-eight postmenopausal women with metastatic breast cancer, in whom estrogen receptors (ER) were positive or unknown, were treated on a controlled trial to determine the effectiveness of tamoxifen and to assess the therapeutic advantage of sequentially adding low-dose cyclophosphamide-methotrexate-5-fluorouracil (CMF) chemotherapy in tamoxifen responders. Patients with known ER negative status were not studied. After the initial 12-week treatment with tamoxifen alone, 60% of ER positive patients achieved complete or partial response as did 35% in whom ER were unknown. Response status further improved in 18% randomized to continue tamoxifen alone vs 31% in whom CMF was added to tamoxifen. There were no statistically significant differences in time to the development of progressive disease or survival between the ER positive and ER unknown patients or between the tamoxifen and tamoxifen plus CMF groups. We conclude that inability to determine ER status should not prejudice against the use of tamoxifen in postmenopausal patients with advanced breast cancer. No benefit has been demonstrated from the addition of CMF chemotherapy in tamoxifen responders.
Similar content being viewed by others
References
McGuire WL: Steroid receptors in human breast cancer. Cancer Res 38: 4289–4291, 1978.
McGuire WL, Carbone PP, Vollmer EP (eds): Estrogen Receptors in Human Breast Cancer. Raven Press, New York, 1975.
Jordan VC: Antitumor activity of antiestrogen ICI 46,474 (tamoxifen) in the dimethylbenzathracene (DMBA)-induced rat mammary carcinoma model. J Steroid Biochem 4: 354, 1974.
Furr BJ, Patterson JS, Richardson DN, Slater SE, Wakeling AE: Tamoxifen,In ME Goldberg (ed): Pharmacological and Biochemical Properties of Drug Substances. American Pharmaceutical Association, Washington, D.C., 1979, Vol 2, pp 355–399.
Cole MP, Jones CTA, Todd IDH: A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI 46474. Br J Cancer 25: 270–275, 1971.
Ward HWC: Anti-oestrogen therapy for breast cancer: A trial of tamoxifen at two dose levels. Br Med J 1: 13–14, 1973.
Kiang DT, Kennedy BJ: Tamoxifen (antiestrogen) therapy in advanced breast cancer. Ann Int Med 87: 687–690, 1977.
Mouridsen H, Palshof T, Patterson J, Battersby L: Tamoxifen in advanced breast cancer. Cancer Treat Rev 5: 131–141, 1978.
Patterson JS, Baum M: Safety of tamoxifen (letter). Lancet i: 105, 1978.
Carbone PP, Tormey DC: Combination chemotherapy for advanced disease,In WL McGuire (ed): Breast Cancer Advances in Research and Treatment. Plenum Medical, New York, 1977, pp 165–215.
Creech RH, Catalano RB, Mastrangelo MJ, Engstrom PF: An effective low-dose intermittent cyclophosphamide, methotrexate, and 5-fluorouracil treatment regimen for metastatic breast cancer. Cancer 35: 1101–1107, 1975.
Bonadonna G and Valagussa P: Dose-response effect of adjuvant chemotherapy in breast cancer. New Engl J Med 304: 10–15, 1981.
McGuire WL: Estrogen receptors in human breast cancer. J Clin Invest 52: 73–77, 1973.
Kaplan EL and Meier P: Nonparamatric estimation from incomplete observations. J Am Statist Assoc 53: 457–481, 1958.
Cox DR: Regression models and life tables. J R Statist Soc (B) 34: 187–220, 1972.
Gehan EA: A generalized Wilcoxon test for comparing arbitrarily single-censored samples. Biometrika 52: 203–223, 1965.
Kennedy BJ: Hormonal therapies in breast cancer. Semin in Oncol 1: 119–130, 1974.
Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, Nichols WC, Cregan ET, Hahn RG, Rubin J, Frytak S: Randomized trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. New Engl J Med 304: 16–20, 1981.
Morgan LR, Posey LE, Krementz ET, Hawley W, Beasley RW: Tamoxifen plus CMF for advanced breast cancer. Proc Am Soc Clin Oncol 18: 308, 1977.
Cavalli F, Jungi F, Martz G, Alberto P, Brunner K: Hormono-chemotherapy followed by chemotherapy in advanced breast cancer. Proc Am Soc Clin Oncol 21: 406, 1980.
Kiang DT, Frenning DH, Gay J, Kennedy BJ: Combination of hormone and chemotherapy in advanced breast cancer. Cancer 47: 452–456, 1981.
Author information
Authors and Affiliations
Additional information
Address for reprints: J.H. Glick, M.D., Hospital of the University of Pennsylvania, 3400 Spruce St., Philadelphia, PA 19104.
Rights and permissions
About this article
Cite this article
Glick, J.H., Creech, R.H., Torri, S. et al. Randomized clinical trial of tamoxifen plus sequential CMF chemotherapy versus tamoxifen alone in postmenopausal women with advanced breast cancer. Breast Cancer Res Tr 1, 59–68 (1981). https://doi.org/10.1007/BF01807893
Issue Date:
DOI: https://doi.org/10.1007/BF01807893