Summary
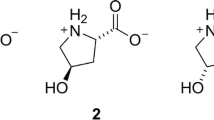
The activity of prolinase (EC 3.4.13.8) was studied in cultured skin fibroblasts derived from three patients with deficient prolidase (EC 3.4.13.9). With pro-val as substrate and manganese in the reaction buffer, prolinase activity was higher in prolidase-deficient cells than in control cells (mean (SEM) 917 (67) nmol min−1 mg−1,n=3, control mean (SEM) 294, (50), n=11). The Michaelis constants were not different for the pro-val and progly substrates in control and prolidase deficient fibroblasts. However, the constants for Vmax rose for both substrates in deficient cells. These results demonstrate that prolinase activity increases in prolidase-deficient fibroblasts as also shown in the plasma of patients with prolidase deficiency. We suggest that in prolidase-deficient fibroblasts, this rise in prolinase activity constitutes an attempt to compensate for the prolidase deficiency by increasing the greatly reduced intracellular proline pool.
Similar content being viewed by others
References
Bio-Rad Technical Bulletin. 1051E, April 1977
Butterworth, J. and Priestman, D. Substrate specificity of manganese-activated prolidase in control and prolidase-deficient cultured skin fibroblasts.J. Inher. Metab. Dis. 7 (1984) 32–34
Butterworth, J. and Priestman, D. Presence in human cells and tissues of two prolidases and their alteration in prolidase deficiency.J. Inher. Metab. Dis. 8 (1985) 193–197
Jackson, S. H. and Heininger, J. A. Proline recycling during collagen metabolism as determined by concurrent18O2- and3H-labelling.Biochim. Biophys. Acta 381 (1975) 359–367
Lemonnier, F., Gautier, M., Wolfrom, C. and Lemonnier, A. Some metabolic differences between human skin and aponeurosis fibroblasts in culture.J. Cell. Physiol. 104 (1980) 415–423
Myara, I. and Stalder, J. F. Plasma prolidase and prolinase activity in prolidase deficiency.Clin. Chem. 32 (1986) 562
Myara, I., Charpentier, C. and Lemonnier, A. Optimal conditions for prolidase assay by proline colorimetric determination: application to iminodipeptiduria.Clin. Chim. Acta 125 (1982) 193–205
Myara, I., Charpentier, C., Wolfrom, C., Gautier, M., Lemmonier, A., Larregue, M., Chamson, A. and Frey, J. In vitro responses to ascorbate and manganese in fibroblasts from a patient with prolidase deficiency and iminodipeptiduria: cell growth, enzyme activity and collagen investigations.J. Inher. Metab. Dis. 6 (1983) 27–31
Myara, I., Charpentier, C. and Lemonnier, A. Prolidase and prolidase deficiency.Life Sci. 34 (1984) 1985–1998
Myara, I., Charpentier, C., Gautier, M. and Lemmonier, A. Cell density affects prolidase and prolinase activity and intracellular amino acid levels in cultured human cells.Clin. Chim. Acta 150 (1985) 1–9
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miech, G., Myara, I., Mangeot, M. et al. Prolinase activity in prolidase-deficient fibroblasts. J Inherit Metab Dis 11, 266–269 (1988). https://doi.org/10.1007/BF01800368
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01800368