Summary
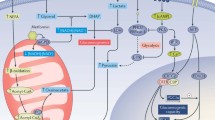
The mechanisms by which the liver maintains a constant supply of oxidizable substrates, which provide energy to the body as a whole, are reviewed. During feeding, the liver builds up energy stores in the form of glycogen and triglyceride, the latter being exported to adipose tissue. During fasting, it releases glucose and ketone bodies. Glucose is formed by degradation of glycogen and by gluconeogenesis from gluconeogenic amino acids provided by muscle. Ketone bodies are produced from fatty acids, released by adipose tissue, and from ketogenic amino acids. The major signals which control the transition between the fed and the fasted state are glucose, insulin and glucagon. These influence directly or indirectly the enzymes which regulate liver carbohydrate and fatty acid metabolism and thereby orient metabolic fluxes towards either energy storage or substrate release. In the fed state, the liver utilizes the energy generated by glucose oxidation to synthesize triglycerides. In the fasted state it utilizes that produced byβ-oxidation of fatty acids to synthesize glucose. The mechanisms whereby a number of inborn errors of glycogen metabolism, of gluconeogenesis and of ketogenesis cause hypoglycaemia are also briefly overviewed.
Similar content being viewed by others
References
Feliu, J. E., Mojena, M. and Lopez-Alarcon, L. Modulation of hepatic pyruvate kinase phosphatase activity.Adv. Prot. Phosphatases 3 (1986) 163–186
Fernandes, J., Saudubray, J.-M. and Tada, K. (eds.),Inborn Metabolic Diseases. Diagnosis and Treatment, Springer-Verlag, Berlin, 1990, 655 pp.
Hagenfeldt, L., von Döbeln, U., Holme, E., Alm, J., Brandberg, G., Enocksson, E. and Lindeberg, L. 3-Hydroxydicarboxylic aciduria — a fatty acid oxidation defect with severe prognosis.J. Pediatr. 116 (1990) 387–392
Häussinger, D. Nitrogen metabolism in liver: structural and functional organization and physiological relevance.Biochem. J. 267 (1990) 281–290
Hers, H. G. Mechanisms of blood glucose homeostasis.J. Inher. Metab. Dis. 13 (1990) 395–410
Hers, H. G. and Van Schaftingen, E. Fructose 2,6-bisphosphate two years after its discovery.Biochem. J. 206 (1982) 1–12
Huang, M.-T. and Veech, R. L. Role of the direct and indirect pathways for glycogen synthesis in rat liver in the postprandial state.J. Clin. Invest. 81 (1988) 872–878
Hue, L. and Rider, M. H. Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues.Biochem. J. 245 (1987) 313–324
Jungermann, K. Metabolic zonation of liver parenchyma: significance for the regulation of glycogen metabolism, gluconeogenesis and glycolysis.Diabetes/Metab. Rev. 3 (1987) 269–293
Katz, J. and McGarry, J. D. The glucose paradox. Is glucose a substrate for liver metabolism?J. Clin. Invest. 74 (1984) 1901–1909
Lane, M. D. and Mooney, R. A. Tricarboxylic acid cycle intermediates and the control of fatty acid synthesis and ketogenesis.Curr. Top. Cell. Regul. 18 (1981) 221–242
McGarry, J. D. and Foster, D. W. Regulation of hepatic fatty acid oxidation and ketone body production.Annu. Rev. Biochem. 49 (1980) 395–420
Quistorff, B. Metabolic heterogeneity of liver parenchymal cells.Essays in Biochemistry 25 (1990) 83–136
Scriver, C. R., Beaudet, A. L., Sly, W. S. and Valle, D. (eds.),The Metabolic Basis of Inherited Disease, 6th ed., McGraw-Hill, New York, 1989, 3006 pp.
Seifter, S. and Englard, S. Energy metabolism. In: Arias, I. M., Jakoby, W. B., Popper, H. Schachter, D. and Shafritz, D. A. (eds.),The Liver: Biology and Pathobiology, 2nd ed., Raven Press, New York, 1988, pp. 279–315
Stalmans, W. The role of the liver in the homeostasis of blood glucose.Curr. Top. Cell. Regul. 11 (1976) 51–97
Sugden, M. C., Holness, M. J. and Palmer, T. N. Fuel selection and carbon flux during the starved-to-fed transition.Biochem. J. 263 (1989) 313–323
Toth, B., Bollen, M. and Stalmans, W. Acute regulation of hepatic protein phosphatases by glucagon, insulin, and glucose.J. Biol. Chem. 263 (1988) 14061–14066
Van den Berghe, G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways.Prog. Biochem. Pharmacol. 21 (1986) 1–32
Van Schaftingen, E. A protein from rat liver confers to glucokinase the property of being antagonistically regulated by fructose 6-phosphate and fructose 1-phosphate.Eur. J. Biochem. 179 (1989) 179–184
Van Schaftingen, E. and Vandercammen, A. Stimulation of glucose phosphorylation by fructose in isolated rat hepatocytes.Eur. J. Biochem. 179 (1989) 173–177
Zakim, D. Metabolism of glucose and fatty acids by the liver. In: Zakim, D. and Boyer, T. D. (eds),Hepatology: A Textbook of Liver Disease, 2nd Edn., W. B. Saunders, Philadelphia, 1900, pp. 65–96
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van den Berghe, G. The role of the liver in metabolic homeostasis: Implications for inborn errors of metabolism. J Inherit Metab Dis 14, 407–420 (1991). https://doi.org/10.1007/BF01797914
Issue Date:
DOI: https://doi.org/10.1007/BF01797914