Summary
-
1.
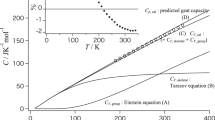
The absolute value of the specific heats of polyethylene, isotactic polypropylene, polyvinyl chloride, poly(tetrafluorethylene), poly(ethylene sebacate), polyethylene terephthalate), copolymers of the latter two, and 6 and 6-6 Nylon are discussed from the standpoint of the number of vibrating units per chain atom. Qualitative agreement is obtained on the assumption that each chain atom has two classical degrees of vibrational freedom.
-
2.
The glass transition temperatures and the increase inc p /T at the glass transition temperature are shown to be functions of the composition of the amorphous regions, magnitude and type of crystallinity and chain orientation.T g is increased andΔ c p /T decreased by fiber orientation whileT g may be increased, decreased or unchanged by crystallization. As the concentration ofB-units in the amorphous regions of anA-B copolymer increases on crystallization,T g decreases, butΔ c p /T per mole of residual melt remains about constant. It is concluded thatT g is more conveniently determined from a cp/T versusT plot than fromH-T curves.
-
3.
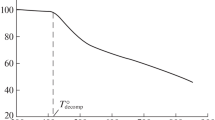
Crystallinity is shown to be of two types, that which is produced under equilibrium conditions,Type I crystallinity, and that which results from random crystallization in the frozen amorphous regions many degrees below the melting point,Type II or “cold” crystallinity. BothType I andType II crystallization can be observed and studied in the 80/20 copolymer of poly(ethylene terephthalate-sebacate).T g is lowered in the case ofType I crystallization, but unchanged in the case ofType II. The melting point ofType II crystals is lowered 30 or 40° from the equilibrium melting point because of the small size of the crystals produced.
-
4.
Cold drawing or chain orientation produces no significant change in the heat content of the polymer provided that no changes in crystallinity occur. The observed values ofΔH for the cold drawing process are thought to be due to changes in crystallinity.
Similar content being viewed by others
References
Smith, C. W. andM. Dole, J. Polymer Sci.20, 37 (1956).
Wunderlich, B. andM. Dole, J. Polymer Sci.24, 201 (1957).
Dole, M. andW. P. Hettinger, Jr., N. R. Larson, andJ. A. Wethington, Jr., J. Chem. Phys.20, 781 (1952).
Marx, P. andM. Dole, J. Amer. Chem. Soc.77, 4771 (1955).
Alford, S. andM. Dole, J. Amer. Chem. Soc.77, 4774 (1955).
Dole, M. andR. M. Hammaker (Unpublished).
Wunderlich, B. andM. Dole, J. Polymer Sci. (In Press.).
Marx, P. andC. W. Smith, A. E. Worthington andM. Dole, J. Phys. Chem.59, 1015 (1955).
Wilhoit, R. C. andM. Dole, J. Phys. Chem.57, 14 (1953).
Pitzer, K. S., J. Chem. Phys.8, 711 (1940).
Edgar, O. B., J. Chem. Soc.1952, 2638.
Raff, R. A. V. andJ. B. Allison, High Polymers-Vol. XI. Polyethylene (New York 1956).
Weir, C. E., J. Res. Nat'l. Bureau of Standards46, 207 (1951).
Parks, W. andR. B. Richards, Trans. Faraday Soc.45, 203 (1949).
Müller, A., Proc. Roy. Soc.178A, 227 (1941).
Stuart, H. A., Die Physik der Hochpolymeren, Bd. III (Berlin-Göttingen-Heidelberg 1955).
Wunderlich, B., Doctoral Dissertation, North-western University (Evanston, Illinois 1957).
Edgar, O. B., J. Chem. Soc.1952, 2638 andE. Ellery, J. Chem. Soc.1952, 2633.
Hoffman, J. D. andJ. J. Weeks (to be published).
Boyer, R. F. andR. S. Spencer, J. Applied Phys.15, 398 (1944).
Ref. (16), p. 648.
Flory, P. J., Trans. Faraday Soc.51, 848 (1955).
See, for example,Flory, P. J. andL. Mandel-kern, J. Polymer Sci.21, 345 (1956).
Flory, P. J., Principles of Polymer Chemistry, p. 570 (Ithaca, N.Y. 1953).
Dole, M., S. Polymer Sci.19, 347 (1956).
See, for example,Epstein, P. S., Textbook of Thermodynamics (New York 1937) p. 216.
Daubeny, R. deP., C. W. Bunn andC. J. Brown, Proc. Roy. Soc.A 226, 531 (1954).
Evans, R. D., H. R. Mighton andP. J. Flory, J. Am. Chem. Soc.72, 2018 (1950).
Dole, M. andB. Wunderlich, J. Polymer Sci.24, 139 (1957).
Müller, F. H. andK. Jäckel, Kolloid-Z.129, 145 (1952).
Müller, F. H. andA. Engelter, Kolloid-Z.149, 126 (1956).
Kolb, H. J. andE. F. Izard, J. Applied Phys.20, 564 (1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dole, M. Thermodynamic properties of high polymers as a function of their pretreatment as determined by specific heat measurements. Kolloid-Zeitschrift 165, 40–57 (1959). https://doi.org/10.1007/BF01797347
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01797347