Summary
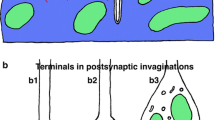
The probability of transmitter secretion from release sites declines along the length of most long terminal branches (> 78 μm) at toad (Bufo marinus) neuromuscular junctions; in contrast, few short terminal branches (< 78 μm) show such a decline. The present study was carried out to see if any of the dimensions of release sites change along the length of terminal branches in a way that can be correlated with the decrease in secretion probability. The size of presynaptic release site structures was determined by examining serial transverse sections through entire terminal branches with the transmission electron microscope; the size of postsynaptic release site structures was determined by examining terminal gutters with the scanning electron microscope after the removal of terminal branches. Long terminal branches showed a significant decrease in the length of their synaptic contact and cross-sectional area (terminal size) with distance from the origin of the branch. In contrast, there was no significant difference in the length of close apposition (< 0.2 μm) between the nerve terminal and postsynaptic muscle membrane; furthermore, neither the length of postsynaptic folds nor the frequency of the folds along the length of the terminal gutter changed. Short terminal branches showed no significant differences in the dimensions of either presynaptic or postsynaptic release site structures. The decline in the length of synaptic contacts whilst the length of close apposition remains relatively constant is due to the progressive encroachment of Schwann cell processes between presynaptic and postsynaptic membranes along the length of long terminal branches.
Similar content being viewed by others
References
Angaut-Petit, D. &Mallart, A. G. (1979) Dual innervation of endplate sites and its consequences for neuromuscular transmission in muscles of adult Xenopus laevis.Journal of Physiology 289, 203–18
Bennett, M. R. &Fisher, C. (1977) The effect of calcium ions on the binomial parameters that control acetylcholine release during trains of nerve impulses at amphibian neuromuscular synapses.Journal of Physiology 271, 673–98.
Bennett, M. R., Fisher, C., Florin, T., Quine, M. &Robinson, J. (1977) The effect of calcium ions and temperature on the binomial parameters that control acetylcholine release by a nerve impulse at amphibian neuromuscular synapses.Journal of Physiology 271, 641–72.
Bennett, M. R. &Florin, T. (1974) A statistical analysis of the release of acetylcholine at newly formed synapses in striated muscle.Journal of Physiology 238, 93–107.
Bennett, M. R., Jones, P. &Lavidis, N. A. (1986a) Transmitter secretion varies between visualized release sites at amphibian neuromuscular junctions.Neuroscience Letters 65, 311–15.
Bennett, M. R., Jones, P. &Lavidis, N. A. (1986b) The probability of quantal secretion along visualized terminal branches at amphibian (Bufo marinus) neuromuscular junction.Journal of Physiology 379, 257–74.
Bennett, M. R. &Lavidis, N. A. (1979) The effect of Ca ions on the secretion of quanta evoked by an impulse at nerve terminal release sites.Journal of General Physiology 74, 429–56.
Bennett, M. R. &Lavidis, N. A. (1982) Variation in quantal secretion at different release sites along developing and mature motor terminal branches.Developmental Brain Research 5, 1–9.
Bennett, M. R. &Raftos, J. (1977) The formation and regression of synapses during the re-innervation of axolotl striated muscles.Journal of Physiology 265, 261–95.
Couteaux, R. &Pecot-Dechavassine, M. (1970) Vesicules synaptiques et poches au niveau de les ‘zones actives’ de la junction neuromusculaire.Comptes rendus hebdomadaire des seances de l'Academic des Sciences 271, 2346–9.
D'Alonzo, A. J. &Grinnell, A. D. (1985) Profiles of evoked release along the length of frog motor nerve terminals.Journal of Physiology 359, 235–58.
Davey, D. F. &Bennett, M. R. (1982) Variation in the size of synaptic contacts along developing and mature motor terminal brances.Developmental Brain Research 5, 11–22.
De Cino, P. (1981) Transmitter release properties along regenerated nerve processes at the frog neuromuscular junction.Journal of Neuroscience 1, 308–17.
Desaki, J. &Uehara, Y. (1981) The overall morphology of neuromuscular junctions as revealed by scanning electron microscopy.Journal of Neurocytology 10, 101–10.
Dreyer, F., Peper, K. M., Akert, D. M., Sandri, C. &Moore, H. (1973) Ultrastructure of the ‘active zone’ in the frog neuromuscular junction.Brain Research 62, 373–80.
Fukunaga, H., Engel, A. G., Osame, M. &Lambert, E. H. (1982) Paucity and disorganization of presynaptic membrane active zones in the Lambert-Eaton myasthenic syndrome.Muscle and Nerve 5, 686–7.
Grinnell, A. D. &Herrera, A. A. (1980) Physiological regulation of synaptic effectiveness at frog neuromuscular junctions.Journal of Physiology 307, 301–17.
Harris, J. B. &Ribchester, R. R. (1979) The relationship between endplate size and transmitter release in normal and dystrophic muscles of the mouse.Journal of Physiology 296, 245–65.
Herrera, A. A., Grinnell, A. D. &Wolowske, B. (1985a) Ultrastructural correlates of naturally occurring differences in transmitter release efficacy in frog motor nerve terminals.Journal of Neurocytology 14, 193–202.
Herrera, A. A., Grinnell, A. D. &Wolowske, B. (1985b) Ultrastructural correlates of experimentally altered transmitter release efficacy in frog motor nerve terminals.Neuroscience 16, 491–500.
Heuser, J. E., Reese, T. S. &Landis, D. M. (1974) Functional changes in frog neuromuscular junction studied with freeze-fracture.Journal of Neurocytology 3, 108–31.
Karnovsky, M. J. (1964) The localization of cholinesterase activity in rat cardiac muscle by electron microscopy.Journal of Cell Biology 23, 217–32.
Katz, B. &Miledi, R. (1977) Transmitter leakage from motor nerve endings.Proceedings of the Royal Society of London, Series B 196, 59–72.
Katz, B. &Miledi, R. (1979) Estimates of quantal content during ‘chemical potentiation’ of transmitter release.Proceedings of the Royal Society of London, Series B 205, 369–78.
Katz, B. &Miledi, R. (1981) Does the motor nerve impulse evoke ‘non-quantal’ transmitter release?Proceedings of the Royal Society of London, Series B 212, 131–7.
Ko, C-P. (1984) Regeneration of the active zone at the frog neuromuscular junction.Journal of Cell Biology 98, 1685–95.
Ko, C-P. (1985) Formation of the active zone at developing neuromuscular junctions in larval and adult bullfrogs.Journal of Neurocytology 14, 487–512.
Ko, C-P., Propst, J. W. &Herrera, A. A. (1985) A comparison of active zone ultrastrucrure in two muscles with markedly different synaptic efficacy.Society for Neuroscience Abstracts 11, 302, 88.4.
Kuno, M., Turkanis, S. A. &Weakley, J. N. (1971) Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog.Journal of Physiology 213, 545–56.
Llinas, R. R. (1982) Calcium in synaptic transmission.Scientific American 247, 56–65.
McMahan, U. J., Spitzer, N. L. &Peper, K. (1972) Visual identification of nerve terminals in living isolated skeletal muscle.Proceedings of the Royal Society of London, Series B 181, 421–30.
Miller, T. M. &Heuser, J. E. (1984) Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction.Journal of Cell Biology 98, 685–98.
Nudell, B. M. &Grinnell, A. D. (1982) Inverse relationship between transmitter release and terminal length in synapses on frog muscle fibres of uniform input resistance.Journal of Neuroscience 2, 216–24.
Nudell, B. M. &Grinnell, A. D. (1982) Regulation of synaptic position, size, and strength in anuran skeletal muscle.Journal of Neuroscience 3, 161–76.
Propst, J. W. &Ko, C-P. (1985) The correlation between synaptic efficacy and active zone ultrastructure studied with freeze fracture of identified neuromuscular junctions.Society for Neuroscience Abstracts 11, 303, 88.5.
Pumplin, D. W. (1983) Normal variations in presynaptic active zones of frog neuromuscular junctions.Journal of Neurocytology 12, 317–23.
Reynolds, E. S. (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy.Journal of Cell Biology 17, 208–12.
Shotton, D. M., Heuser, J. E., Reese, B. F. &Reese, T. S. (1979) Post-synaptic membrane folds of the frog neuromuscular junction visualized by scanning electron microscopy.Neuroscience 4, 427–35.
Spurr, A. R. (1969) A low viscosity epoxy resin embedding medium for electron microscopy.Journal of Ultrastructure Research 26, 31–43.
Torri-Tarelli, F., Grohovaz, F., Fesce, R. &Ceccarelli, B. (1985) Temporal coincidence between synaptic vesicle fusion and quantal secretion of acetylcholine.Journal of Cell Biology 101, 1386–99.
Tremblay, J. D., Robitaille, R. &Grenon, G. (1984) Distribution of spontaneous release along the frog neuromuscular junction.Neuroscience Letters 51, 247–52.
Triller, A. &Korn, H. (1982) Transmission at a central inhibitory synapse. III. Ultrastructure of physiologically identified and stained terminals.Journal of Neurophysiology 48, 708–36.
Verma, V. &Reese, T. S. (1984) Structure and distribution of neuromuscular junctions on slow muscle fibres in the frog.Neuroscience 12, 647.
Walrond, J. P. &Reese, T. S. (1985) Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers.Journal of Neuroscience 5, 1118–31.
Werle, M. J., Herrera, A. A. &Grinnell, A. D. (1984) Ultrastructural uniformity along branches of frog motor nerve terminals.Neuroscience Abstracts 10, 919.
Wernig, A. (1975) Estimates of statistical release parameters from crayfish and frog neuromuscular junctions.Journal of Physiology 244, 107–221.
Yoshikami, D. &Okun, L. M. (1984) Staining of living presynaptic nerve terminals with selective fluorescent dyes.Nature 310, 53–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bennett, M.R., Lavidis, N.A. & Armson, F.M. Changes in the dimensions of release sites along terminal branches at amphibian neuromuscular synapses. J Neurocytol 16, 221–237 (1987). https://doi.org/10.1007/BF01795306
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01795306