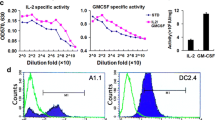
Summary
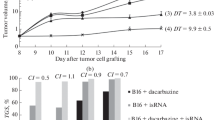
Incubation of C3H/Hen thymocytes in the presence of recombinant human tumor necrosis factor α (TNF) and interleukin-2 (IL-2) augmented the generation of antibody-dependent cellular cytotoxicity (ADCC) when compared to cells cultured in TNF or IL-2 alone. This effect was optimal when 100–200 units/ml IL-2 was used together with 103–104 units/ml TNF. TNF alone at any concentration could not mediate the induction of ADCC. Similar to the results obtained in vitro, TNF, when given alone, had no effect on the generation of ADCC in vivo. The addition, however, of TNF to IL-2, given at 10 000 and 20 000 but not 40 000 units, enhanced the IL-2-induced ADCC on a per-cell basis. Furthermore, TNF enhanced the total ADCC activity in various organs including the liver, spleen and thymus as a result of an increase in the number of mononuclear cells isolated from these organs. The increase in total ADCC activity was optimal when 110 000–220 000 units (5–10 µg) TNF were employed together with IL-2. The combined treatment with TNF and IL-2 also increased the intracellular benzyloxycarbonyl-l-l-lysinethiobenzyl-ester esterase content in cells isolated from the livers of mice treated with these cytokines. On the basis of these results we treated mice bearing a single B 16 melanoma nodule with TNF and TNF + IL-2 given with or without anti-B 16 monoclonal antibody. We found that TNF administration augmented the anti-tumor effect of specific anti-B 16 antibodies, and the addition of IL-2 further increased this anti-tumor effect.
Similar content being viewed by others
References
Asher AL, Mule JJ, Reichert CM, Shiloni E, Rosenberg SA (1987) Studies of the antitumor efficacy of systemically administered recombinant tumor necrosis factor against several murine tumors in vivo. J Immunol 138: 963
Aguet M, Vignaux F, Fridman WH, Gresser I (1987) Enhancement of Fcγ receptor expression in interferon-treated mice. Eur J Immunol 11: 926
Balkwill FR, Lee A, Aldman G, Moodie E, Thomas A, Tavernier J, Fiers W (1986) Human tumor xenografts treated with recombinant human tumor necrosis factor alone or in combination with interferons. Cancer Res 46: 3990
Creasy AA, Reynolds TR, Laird W (1986) Cures and partial regression of murine and human tumors by recombinant tumor necrosis factor. Cancer Res 46: 5687
Eisenthal A, Rosenberg SA (1989) The effect of various cytokines on the in vitro induction of antibody-dependent cellular cytotoxicity in murine cells. Enhancement of IL-2 induced antibody-dependent cellular cytotoxicity activity by IL-1 and tumor necrosis factor-α. J Immunol 142: 2307
Eisenthal A, Lafreniere R, Lefor AT, Rosenberg SA (1987) The effect of anti-B 16 melanoma monoclonal antibody on established murine B 16 melanoma liver metastases. Cancer Res 47: 2771
Eisenthal A, Shiloni E, Rosenberg SA (1988) Characterization of IL-2 induced murine cells which exhibit ADCC activity. Cell Immunol 115: 257
Ezekovirtz AB, Bampton M, Gordon S (1983) Macrophage activation selectively enhances expression of Fc receptors for IgG2a. J Exp Med 157: 807
Gehan EA (1965) Generalized Wilcoxon test for comparing arbitrarily single censored samples. Biometrica 52: 203
Graziano FR, Franger MW (1987) Fc γRs and Fc γRII on monocytes and granulocytes are cytotoxic trigger molecules for tumor cells. J Immunol 139: 3536
Handwerger BS, Koren HS (1976) The nature of the effector cell in antibody-dependent, cell mediated cytolysis (ADCC): the cytotoxic activity of murine cells and peritoneal macrophages. Clin Immunol Immunopathol 5: 272
Henkart P, Yue CC (1988) The role of cytoplasmic granules in lymphocyte cytotoxicity. Prog Allergy 40: 82
Hori K, Ehrke MJ, Mace K, Maccubbin D, Doyle MJ, Otsuka Y, Mihich E (1987) Effect of recombinant human tumor necrosis factor on the induction of murine macrophage tumoricidal activity. Cancer Res 47: 2793
Hosnik C, Jung G, Reisfeld RA (1986) Lymphokine-activated killer cells targeted by monoclonal antibodies to the disialogangliosides GD2 and GD3 specifically lyse human effector cells of neuroectodermal origin. Proc Natl Acad Sci USA 83: 7893
Huddlestone JR, Merigan TC, Oldstone MB (1979) Induction and kinetics of natural killer cells in humans following interferon therapy. Nature 282: 417
Lopez AF, Nicola NA, Burgess AW, Metcalf D, Battye FL, Sewell WA, Vadas M (1983) Activation of granulocyte cytotoxic function by purified mouse colony-stimulating factors. J Immunol 131: 2983
McIntosh JK, Mule JJ, Krosnick JA, Rosenberg SA (1989) Combination cytokine immunotherapy with tumor necrosis factor α, interleukin-2 and α-interferon and its synergistic antitumor effects in mice. Cancer Res 49: 1408
Mule JJ, Krosnick JA, Rosenberg SA (1989) IL-4 reulgation of murine lymphokine-activated killer (LAK) activity in vitro. Effect on the interleukin-induced expansion, cytotoxicity and phenotype of LAK effectors. J Immunol 142: 726
Munn DH, Cheung NKV (1987) Interleukin-2 enhancement of monoclonal antibody-mediated cellular cytotoxicity against human melanoma. Cancer Res 47: 6600
Ortaldo JR, Woodhouse C, Morgan AC, Herberman RB, Cheresh DA, Reisfeld RA (1987) Analysis of effector cells in human antibody dependent cellular cytotoxicity with murine monoclonal antibodies. J Immunol 138: 3566
Owen-Schaub LB, Gutterman JU, Grimm EA (1988) Synergy of tumor necrosis factor and interleukin 2 in the activation of human cytotoxic lymphocytes: effect of tumor necrosis factor α and interleukin 2 in the generation of human lymphokine-activated killer cell cytotoxicity. Cancer Res 48: 788
Pasternack MS, Verret CR, Liu MA, Eisen HN (1986) Serine esterase in cytolytic T lymphocytes. Nature 322: 740
Philip R, Epstein LB (1986) Tumor necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, γ-interferon and interleukin-1. Nature 323: 86
Ralph P, Nakoinz I, Rennick D (1988) Role of interleukin 2, interleukin 4 and α, β and γ interferon in stimulating macrophage antibody-dependent tumoricidal activity. J Exp Med 167: 712
Ruff MR, Gifford GE (1980) Purification and physico chemical characterization of rabbit tumor necrosis factor. J Immunol 125: 1671
Sachs DH, Cone JL (1973) A murine B-cell aloantigen determined by gene(s) linked to the major histocompatbility complex. J Exp Med 138: 1289
Santoni A, Herberman RB, Holden HT (1979) Correlation between natural and antibody-dependent cell-mediated cytotoxicity against tumor targets in the mouse. II. Characterization of the effector cells. J Natl Cancer Inst 63: 995
Scheurich P, Thomas B, Ucer U, Pfizemaier K (1987) Immunotrgulatory activity of recombinant human tumor necrosis factor (NF)-α: induction of TNF receptors on human T cells and TNf-α, mediated enhancement of T cell responses. J Immunol 138: 1786
Shiloni E, Eisenthal A, Sachs D, Rosenberg SA (1987) Antibody-dependent cellular cytotoxicity mediated by murine lymphocytes activated in recombinant interleukin-2. J Immunol 138: 1992
Titus JA, Finkelman FD, Stephany DA, Jones JF, Segal DM (1984) Quantitative analysis of Fc receptors on murine spleen cell populations by using dual parameter flow cytometry. J Immunol 133: 556
Unkeles JC (1979) Characterization of a monoclonal antibody directed against mouse macrophages and lymphocyte Fc receptors. J Exp Med 150: 580
Weiner LM, Steplewski Z, Koprowski H, Litwin S, Comis RL (1988) Divergent dose-related effects of gamma-interferon therapy on in vitro antibody-dependent cellular and non specific cytotoxicity by human peripheral blood monocytes. Cancer Res 48: 1042
Wiltrout RH, Santoni A, Peterson ES, Knott DC, Overton WR, Herberman RB, Holden HT (1985) Reactivity of anti-asialo GM1 serum with tumoricidal and non-tumoricidal mouse macrophages. J Leukocyte Biol 37: 597
Zagury D, Morgan DA, Fouchard M, Bernard J, Feldman M, Herberman RB (1985) Heterogeneity of human natural killer cell. J Natl Cancer Inst 74: 553
Author information
Authors and Affiliations
Additional information
Offprint requests to: S. A. Rosenberg, Surgery Branch, National Cancer Institute, Building 10, Room 2B42, Bethesda, MD 20892 USA
Rights and permissions
About this article
Cite this article
Eisenthal, A., McIntosh, J.K. Effect of recombinant human tumor necrosis factor α on the induction of antibody-dependent cellular cytotoxicity in the treatment of established B16 melanoma liver nodules. Cancer Immunol Immunother 31, 243–249 (1990). https://doi.org/10.1007/BF01789176
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01789176