Summary
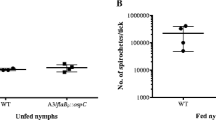
ABorrelia garinii isolate (NE11H) was obtained from the hemolymph of infedIxodes ricinus. NE11H expressed four major proteins of 33 kDa, 32 kDa, 23 kDa and 22 kDa. Duringin vitro culture, NE11H successively lost the expression of the 22 kDa and 23 kDa proteins and the NE11H variant (NE11Hp15) was not recognized by an immune serum specific for the OspC protein (anti-OspC IS). However, when reintroduced into tick midguts, NE11Hp15 spirochetes present in the midgut again reacted with anti-OspC IS. A clone derived from the wild type line, cNE11H, lacked the 22 kDa but not the 23 kDa protein. The 23 kDa protein of cNE11H was recognized by anti-OspC IS. In addition, the two descendant lines (NE11Hp15 and cNE11H) lost their capacity to induce clinical arthritis in SCID mice. When cNE11H was reintroduced into ticks and reisolated from various tick organs, most reisolates presented the same reaction with anti-OspC IS as cNE11H. Interestingly, two reisolates obtained from the tick midgut reexpressed large amounts of the 22 kDa protein which was recognized by anti-OspC IS and these two reisolates induced clinical arthritis in SCID mice. The results confirm that proteins of 22/23 kDa are differentially expressed duringin vitro subcultures and in ticks, and show that proteins which are not detectable afterin vitro culture may be reexpressed after reexposure ofB. burgdorferi to its former environment in the tick. The data suggest that the pathogenicity ofB. burgdorferi for mice might be influenced by environmental factors via differential expression of 22/23 kDa proteins.
Zusammenfassung
EinBorrelia garinii Isolat (NE11H) wurde aus der Hämolymphe nicht gefütterterIxodes ricinus Zecken gewonnen. NE11H exprimierte vier Hauptproteine mit einem Molekulargewicht von jeweils 33, 32, 23 und 22 kDa. Im Laufe derin vitro Kultur entstand eine Variante (NE11Hp15), die durch den Verlust der 22 und 23 kDa Proteine gekennzeichnet war. NE11Hp15 zeigte keine Reaktion mit einem Immunserum gegen OspC. Wurde NE11Hp15 in Zecken passagiert, fand sich wieder eine Reaktion mit dem anti-OspC-Immunserum. Ein Klon der Ausgangslinie NE11H (cNE11H) war durch das Fehlen des 22 kDa, nicht jedoch des 23 kDa Proteins, gekennzeichnet. Das 23 kDa Protein von cNE11H wurde durch anti-OspC-Immunserum erkannt. Sowohl NE11Hp15 als auch cNE11H waren nicht in der Lage, eine klinisch nachweisbare Arthritis in SCID-Mäuse zu induzieren. Nach Reinokulation von cNE11H in Zecken und anschließender Reisolation aus verschiedenen Zeckenorganen, zeigten die meisten Reisolate eine Reaktion mit anti-OspC-Immunserum wie der Ausgangsklon cNE11H. Interessanterweise fand sich bei zwei Reisolaten aus dem Zeckenmitteldarm eine starke Expression des 22 kDa Proteins, welches durch das anti-OspC-Immunserum erkannt wurde. Darüberhinaus induzierten diese beiden Reisolate eine klinisch nachweisbare Arthritis in SCID-Mäusen. Zusammengefaßt zeigen unsere Resultate, daß die spirochätalen 22/23 kDa Proteine während der Zeckenpassage differentiell exprimiert werden. Darüberhinaus zeigen sie auch, daß spirochätale Proteine, die nach Kultivierungin vitro nicht mehr nachweisbar sind, dann reexprimiert werden können, wennB. burgdorferi der Mikroumgebung des Zeckenorganismus ausgesetzt wird. Wir postulieren, daß die Pathogenität vonB. burgdorferi in Mäusen durch umgebungsbedingte differentielle Expression der 22 und 23 kDa Proteine beeinflußt werden könnte.
Similar content being viewed by others
References
Burgdorfer, W., Barbour, A. G., Hayes, S. F., Benach, J. L., Grunwaldt, E., Davis, J. P. Lyme disease — a tick-borne spirochetosis? Science 216 (1982) 1317–1319.
Burgdorfer, W., Barbour, A. G., Hayes, S. F., Péter, O., Aeschlimann, A. Erythema chronicum migrans — a tick borne spirochetosis. Acta Trop. 40 (1983) 79–83.
Gern, L., Zhu, Z., Aeschlimann, A. Development ofBorrelia burgdorferi inIxodes ricinus female during blood feeding. Ann. Parasitol. Hum. Comp. 65 (2) (1990) 89–93.
Ribeiro, J. M. C., Mather, T. N., Piesman, J., Spielman, A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J. Med. Entomol. 24 (2) (1987) 201–205.
Zung, J. L., Lewengrub, S., Rudzinska, M. A., Spielman, A., Telford III, S. R., Piesman, J. Fine structural evidence for the penetration of the Lyme disease spirocheteBorrelia burgdorferi through the gut and salivary tissues ofIxodes dammini. Can. J. Zool. 67 (1989) 1737–1990.
Leuba-Garcia, S., Kramer, M. D., Wallich, R., Gern, L. Characterization ofBorrelia burgdorferi isolated from different organs ofIxodes ricinus ticks collected in nature. Zentralbl. Bakt. Hyg. 280 (1994) 468–475.
Lebet, N., Gern, L. Histological examination ofBorrelia burgdorferi infections in unfedIxodes ricinus nymphs. Exp. Appl. Acarol. 18 (3) (1994) 177–183.
Hu, C. M., Leuba-Garcia, S., Aeschlimann, A., Kramer, M. D., Gern, L. Comparison in the immunological properties ofBorrelia burgdorferi isolates fromIxodes ricinus derived from three endemic areas in Switzerland. Epidemiol. Infect. 112 (1994) 533–542.
Péter, O., Bretz, A. G. Polymorphism of outer surface proteins ofBorrelia burgdorferi as a tool for classification. Zentralbl. Bakt. Hyg. 277 (1992) 28–33.
Baranton, G., Postic, D., Saint Girons, I., Boerlin, P., Piffaretti, J. C., Assous, M., Grimont, P. A. D. Delineation ofBorrelia burgdorferi sensu stricto,Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42 (3) (1992) 378–383.
Canica, M. M., Nato, F., Du Merle, L., Mazie, J. C., Baranton, G., Postic, D. Monoclonal antibodies for identification ofBorrelia afzelii sp. nov., associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 25 (4) (1993) 441–448.
Wilske, B., Preac Mursic, V., Jauris, S., Hofmann, A., Pradel, I., Soutschek, E., Schwab, E., Will, G., Wanner, G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein ofBorrelia burgdorferi. Infect. Immun. 61 (1993) 2182–2191.
Wilske, B., Preac Mursic, V., Schierz, G., Kühbeck, R., Barbour, A. G., Kramer, M. D. Antigenic variability ofBorrelia burgdorferi. Ann. N. Y., Acad. Sci. 539 (1988) 126–143.
Schwan, T. G., Burgdorfer, W., Garon, C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochetesBorrelia burgdorferi, as a result ofin vitro cultivation. Infect. Immun. 56 (1988) 1831–1836.
Moody, K. D., Barthold, S. W., Terwilliger, G. A. Lyme borreliosis in laboratory animals: effect of host species andin vitro passage ofBorrelia burgdorferi. Am. J. Trop. Med. Hyg. 43 (1) (1990) 87–92.
Hu, C. M., Gern, L., Aeschlimann, A. Changes in the protein profile and antigenicity of differentBorrelia burgdorferi strains after reintroduction toIxodes ricinus. Parasite Immunol. 14 (1992) 415–427.
Gern, L., Hu, C. M., Toutoungi, L. N., Kramer, M.: Antigenic variation inBorrelia burgdorferi after passage throughIxodes ricinus andIxodes hexagonus. In: Proceedings of the First International Conference on Tick-Borne Pathology Host-Vector Interface. St. Paul, USA 1992, pp. 121–125.
Barbour, A. G. Isolation and cultivation of Lyme spirochete. Yale J. Biol. Med. 57 (1984) 521–525.
Bundoc, V. G., Barbour, A. G. Clonal polymorphisms of outer membrane protein OspB ofBorrelia burgdorferi. Infect. Immun. 57 (1989) 2733–2741.
Monin, R., Gern, L., Aeschlimann, A. A study of the modes of transmission ofBorrelia burgdorferi byIxodes ricinus. Zentralbl. Bakt. Hyg. (Suppl. 18) (1989) 14–20.
Gern, L., Toutoungi, L. N., Hu, C. M., Aeschlimann, A. Ixodes (Pholeoixodes) hexagonus, an efficient vector ofBorrelia burgdorferi in the laboratory. Med. Vet. Entomol. 5 (1991) 431–435.
Schwan, T. G., Schrumpf, M. E., Karstens, R. H., Clover, J. R., Wong, J., Daugherty, M., Struthers, M., Rosa, P. A. Distribution and molecular analysis of Lyme disease spirochetes,Borrelia burgdorferi, isolated from ticks throughout California. J. Clin. Microbiol. 31 (1993) 3096–3108.
Barbour, A. G., Schrumpf, M. E. Polymorphisms of major surface protein ofBorrelia burgdorferi. Zentralbl. Bakt. Hyg. (A) 263 (1986) 83–91.
Kramer, M. D., Schaible, U. E., Wallich, R., Moter, S. E., Petzoldt, D., Simon, M. M. Characterization ofBorrelia burgdorferi associated antigens by monoclonal antibodies. Immunobiol. 181 (1990) 357–366.
Schaible, U. E., Gay, S., Museteanu, C., Kramer, M. D., Zimmer, G., Eichmann, K., Museteanu, U., Simon, M. M. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am. J. Pathol. 137 (1990) 811–820.
Schaible, U. E., Wallich, R., Kramer, M. D., Gern, L., Anderson, J. F., Museteanu, C., Simon, M. M. Immune sera to individualBorrelia burgdorferi isolates or recombinant OspA thereof protect SCID mice against infection with homologous strains but only partially or not at all against those of different OspA/OspB genotype. Vaccine 11 (1993) 1049–1054.
Schwan, T. G., Burgdorfer, W. Antigenic changes ofBorrelia burgdorferi as a result ofin vitro cultivation. J. Infect. Dis. 156 (5) (1987) 852–853.
Rosa, P. A., Hogan, D. M.: Colony formation byBorrelia burgdorferi in solid medium clonal analysis ofOsp locus variants. In: Proceedings of the First International Conference Tick-Borne Pathology Host-Vector Interface. St. Paul, USA 1992 pp. 95–103.
Masuzawa, T., Kurita, T., Kavabata, H., Yanagihara, Y. Relationship between infectivity and OspC expression in Lyme diseaseBorrelia. FEMS Microbiol. Lett. 123 (1994) 319–324.
Schwan, T. G., Piesman, J., Golde, W. T., Dolan, M. C., Rosa, P. A. Induction of an outer surface protein onBorrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92 (1995) Microbiology pp. 2909–2913.
Ma, B., Christen, B., Leung, D., Vigo-Pelfrey, C. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies againstBorrelia burgdorferi. J. Clin. Microbiol. 30 (1992) 370–376.
Wilske, B., Preac Mursic, V., Schierz, G., Liegl, G., Gueye, W. Detection of IgM and IgG antibodies toBorrelia burgdorferi using different strains as antigen. Lyme borreliosis II, Zentralbl. Bakt. Hyg. (Suppl. 77) (1989) 299–309.
Gern, L., Schaible, U. E., Simon, M. M. Mode of inoculation of the Lyme disease agentBorrelia burgdorferi influences infection and immune responses in inbred strains of mice. J. Infect. Dis. 167 (1993) 971–975.
Margolis, N., Hogan, D., Tilly, K., Rosa, P. A. Plasmid location ofBorrelia purine biosynthesis gene homologues. J. Bacteriol. 176 (1994) 6427–6432.
Kurtti, T. J., Munderloh, U. G., Krueger, D. E., Johnson, R. C., Schwan, T. G. Adhesion to and invasion of cultured tick (Acarina: Ixodidae) cells byBorrelia burgdorferi (Spirochaetales: Spirochaetaceae) and maintenance of infectivity. J. Med. Entomol. 30 (3) (1993) 586–596.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hu, C.M., Gern, L., Simon, M. et al. Tick factors andin vitro cultivation influence the protein profile, antigenicity and pathogenicity of a clonedBorrelia garinii isolate fromIxodes ricinus hemolymph. Infection 24, 251–257 (1996). https://doi.org/10.1007/BF01781105
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01781105