Summary
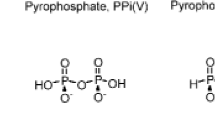
Organic pyrophosphates such as UppA and NAD are formed when a solution containing a nucleotide, a nucleoside 5′-polyphosphate, Mg2+ and imidazole are allowed to dry out. We suggest that this synthesis may have occured concurrently with oligonucleotide formation.
Similar content being viewed by others
Abbreviations
- Im:
-
Imidazole
- CDI:
-
1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride
- EDTA:
-
ethylenediaminetetraacetic acid
- A:
-
adenosine
- U:
-
uridine
- pnA:
-
adenosine 5′-poly-phosphate containing n phosphate residues
- pU:
-
uridine 5′-phosphate
- AppA:
-
P1,P2-diadenosine 5′-pyrophosphate
- UppA:
-
P1-(uridine 5′)-P2-(adenosine 5′)-pyrophosphate
- ImpA:
-
adenosine 5′-phosphorimidazolide
- NMN:
-
nicotinamide mononucleotide
- NAD:
-
nicotinamide-adenine dinucleotide
References
Lehninger, A.L. (1975). In: Biochemistry, 2nd ed. Chapter37, 1040, New York: Worth Publishers, Inc.
Lohrmann, R. (1975). J. Mol. Evol.6, 237
Lohrmann, R. (1976). J. Mol. Evol.8, 197
Lohrmann, R. (1977). J. Mol. Evol.10, 137
Lohrmann, R., Orgel, L.E. (1973). Nature244, 418
Lohrmann, R., Ranganathan, R., Sawai, H., Orgel, L.E. (1975) J. Mol. Evol.5, 57
Lowenstein, J.M. (1958). Biochem. J.70, 222
Lowenstein, J.M. (1960). Biochem. J.75, 209
Orgel, L.E. (1968). J. Mol. Biol.38, 381
Orgel, L.E., Lohrmann, R. (1974). Accounts of Chem. Res.7, 368
Orgel, L.E., Sulston, J.E. (1971). In: Prebiotic and Biochemical Evolution, A.P. Kimball and J. Orô eds., pp. 89–94, Amsterdam-London: North-Holland Publ. Comp.
Sawai, H., Lohrmann, R., Orgel, L.E. (1975) J. Mol. Evol.6, 165
Sawai, H., Orgel, L.E. (1975). J. Mol. Evol.6, 185
Smith, M., Khorana, H.G. (1958). J. Am. Chem. Soc.80, 1141
White, H.B. (1976). J. Mol. Evol.7, 101
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lohrmann, R., Orgel, L.E. Formation of P1, P2-dinucleoside 5′-pyrophosphates under potentially prebiological conditions. J Mol Evol 11, 17–23 (1978). https://doi.org/10.1007/BF01768021
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01768021