Summary
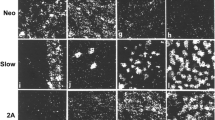
Myoid cells of calf and rat thymus have been identified by staining with a monoclonal antibody to the heavy chain of myosin that is not isoform specific. Heterogeneity in the protein composition of myoid cells has been demonstrated by staining with antibodies to the skeletal muscle isoforms of the myosin heavy chain, C-protein and components of the troponin complex. The immunochemical studies suggest that the myoid cells contain proteins closely resembling if not identical with those present in the myofibrils of skeletal muscle. The slow and fast skeletal muscle isoforms of the myofibrillar proteins are present in a large proportion of the myoid cells. A fraction of the myoid cells contains only the fast isoforms of the myofibrillar proteins but there is no sharp compartmentalization of the isoforms as occurs in type 1 and type 2 fibres of skeletal muscle. In general the pattern of gene expression is similar to that of developing skeletal muscle.
Similar content being viewed by others
References
deBlas, A. L. &Cherwinski, H. M. (1983) Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies.Analyt. Biochem. 133, 214–19.
Dhoot. G. K., Frearson, N. &Perry, S. V. (1979) Polymorphic forms of troponin T and troponin C and their localization in striated muscle cell types.Expl Cell Res. 122, 339–50.
Dhoot, G. K., Gell, P. G. H. &Perry, S. V. (1978) The localization of the different forms of troponin I in skeletal and cardiac muscle cells.Expl Cell Res. 117, 357–70.
Dhoot, G. K., Hales, M. C., Grail, B. M. &Perry, S. V. (1985) The isoforms of C protein and their distribution in mammalian skeletal muscle.J. Musc. Res. Cell Motility 6, 487–505.
Drury, R. A. B. &Wallington, E. A. General staining procedures. InCarleton's Histological Technique, pp. 114–37. New York, Toronto: Oxford University Press.
Grand, R. J. A., Perry, S. V. &Weeks, R. A. (1979) Troponin C-like proteins from smooth muscles and other tissues.Biochem. J. 177, 521–9.
Hammar, J. A. (1905) Zur Histogenese und Involation der Thymus druse.Anat. Auz. 27, 23–30, 41–89.
Hancock, K. &Tsang, V. C. W. (1983) Indian ink staining of proteins on nitrocellulose paper.Analyt. Biochem. 133, 157–62.
Hayward, A. R. (1972) Myoid cells in the human foetal thymus.J. Path. 106, 45–8.
Köhler, G. &Milstein, C. (1975) Continuous culture of fused cells secreting specific antibody.Nature, Land. 256, 459–7.
Lowe, J., Hardie, D., Jefferis, R., Ling, N. R., Drysdale, P., Richardson, P., Raykundalia, C, Catty, D., Appleby, P., Drew, R. &Maclennan, I. C. (1981) Properties of monoclonal antibodies to human immunoglobulin kappa and lambda chains.Immunology 42, 649–59.
Mackay, I. R. &Goldstein, G. (1967) Thymus and muscle.Clin. exp. Immun. 2, 139–40.
Mayer, S. (1888) Zur Lehr von der Schild-drusse und Thymus bei den Amphibiin.Anat. Auz. 97–103.
Perry, S. V. (1955) Myosin adenosinetriphosphatase. InMethods in Enzymology, Vol. 2. (edited byColowick, S. P. &Kaplan, N. O. pp. 582–8. New York: Academic Press.
Perry, S. V. &Cole, H. A. (1974) Phosphorylation of troponin and effects of interactions between the components of the complex.Biochem. J. 141, 733–43.
Pires, E., Perry, S. V. &Thomas, M. A. W. (1974) Myosin light chain kinase, a new enzyme from striated muscle.FEBS Lett. 41, 292–6.
Raviola, E. &Raviola, G. (1967) Striated muscle cells in the thymus of reptiles and birds.Am. J. Anat. 121, 623–46.
Rimmer, J. J. (1980) Myoid cells and Myasthenia Gravis.Dev. comp. Immun. 4, 385–94.
Syska, H., Perry, S. V. &Trayer, I. P. (1974) A new method of preparation of troponin I (inhibitory protein) using affinity chromatography. Evidence for three different forms of troponin I in striated muscle.FEBS Lett. 40, 253–7.
Terni, T. (1929) Ricerchi istologische sullinnervazione del timo dei sauropsidi.Z. Zeilforsch. 9, 377–424.
Towbin, H., Staehelin, T. &Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications.Proc. natn. Acad. Sci., U.S.A. 76 4350–4.
Toro, I., Olah, I., Ruhlich, P. &Viragh, S. Z. (1969) Electron microscopic observations on myoid cells in the frog thymus.Anat. Rec. 165, 329–42.
Van Der Geld, H., Feldkamp, T. E. W., Van Loghem, J. J. &Oosterhuis, H. J. G. (1964) Reactivity of myasthenia gravis serum gammaglobulin with skeletal muscle and thymus demonstrated by immunofluorescence.Proc. Soc. Exp. Biol. Med. 115, 782–5.
Weeds, A. G. (1976) Light chains of myosin.Eur. J. Biochem. 66,157–73.
Weeds, A. G. &Taylor, R. S. (1975) Separation of S1 isoenzymes from skeletal muscle.Nature, Lond. 257, 54–6.
Wray, W., Boulikas, T., Wray, V. P. &Hancock, R. (1981) Silver staining of protein in polyacrylamide gels.Analyt. Biochem. 118, 197–203.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dhoot, G.K., Dransfield, I., Grand, R.J.A. et al. Distribution of isoforms of the myofibrillar proteins in myoid cells of thymus. J Muscle Res Cell Motil 7, 351–360 (1986). https://doi.org/10.1007/BF01753656
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01753656