Summary
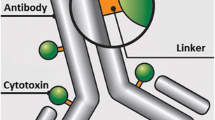
Measurements in cancer patients showed that the pH of tumors averages 0.8 unit lower than that of the surrounding normal tissues, confirming published work. Based on this, the anti-carcinoma monoclonal antibody (mAb) L6 was used to prepare immunoconjugates with daunomycin (DM), the drug being released at the acidic pH of the tumor. A direct linking of the aconitic derivative of DM (AcoDM) to mAb L6 led to conjugates that either had a low drug/antibody ratio (<5:1) or precipitated in vitro. In order to increase the drug load and avoid precipitation, several biopolymers were tested as spacers between the drug and the L6. To attach the polymer derivative to the mAb, the former was maleimidized and the mAb was thiolated. The AcoM/mAb ratio obtained was 20, and the mAb retained its highly specific binding to tumor cells. At pH 6 the AcoDM-L6 conjugate was toxic to cultured C-3347 carcinoma cells with an inhibitory concentration (IC50) of 5 µg/ml. The conjugate was less effective than the free DM with an IC50 of 0.2 µg/ml. The L6 alone was not toxic. At a tumor pH of 6.5, 15% of the AcoDM was released. The amount of released drug reached a maximum 24–48 h after exposure to the acidic medium.
In vivo localization studies demonstrated a similar tumor uptake of the conjugate and mAb L6 with 18% of the injected dose/g tumor and a maximum uptake in tumor 48 h after injection. Our data indicate that it is possible to construct conjugates based on a pH-sensitive linker that can be targeted successfully to a tumor with release of a portion of the drug at the tumor site, but testing is needed to establish whether such release has anti-tumor activity in vivo and offers an advantage over treatment with unconjugated drug.
Similar content being viewed by others
References
Arnold IJ Jr (1985) Polylysine-drug conjugates. Methods Enzymol 112: 270–285
Arnold IJ Jr, Dagan A, Gutheil JC, Kaplan NO (1979) Antineoplastic activity of polylysine with some ascites tumor cells. Proc Natl Acad Sci, USA 76: 3246–3250
Ashby BS, Cantab MB (1966) pH studies in human malignant tumors. Lancet 2: 312–315
Beaumier PL, Neuzil D, Yang H-M, Noll EA, Krohn K, Hellstrom I, Hellstrom KE, Nelp W (1986) Immunoreactivity assay for labeled anti-melanoma monoclonal antibodies. J Nucl Med 27: 824–828
Bradford MD (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–284
Bumol TF, Laguzza BC, Dehardt SV. (1976) KS1/4-4 deacetyl vinblastine-3-carbohydrazide (KS1/4 DAVLB-Hydrazine): preclincal studies on monoclonal antibody drug conjugate for site directed therapy of human adenocarcinoma. Annu Meet Am Assoc Cancer Res 28: 393
Croce AC, Prosperi E, Supino R, Bottiroli G (1986) Anthracyclineinduced inhibition of membrane permeability function dependent on metabolic energy. Br J Cancer 54: 943–950
Deen WM, Satvat B (1981) Determinations of the glumerular filtration of proteins. Am J Physiol F162–F170
Diener E, Diner UE, Sinha A, Xie S, Vergidis R (1986) Specific immunosuppression by immunotoxinl containing daunomycin. Science 231: 148–150
Dillman RA, Shawles DL, Johnson DE, Myer DL, Koziol JA, Frincke JM (1986) Preclinical trials with combinations and conjugates of T101 monoclonal antibody and doxorobucin. Cancer Res 46: 4886–4891
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem, Biophys 82: 70–77
FitzGerald DJ, Willingham MC, Pastan I (1986) Antitumor effects of an immunotoxin made withPseudomonas exotoxin in a nude mouse model of human ovarian cancer. Proc Natl Acad Sci USA 83: 6627–6630
Garrigues HJ, Tilgen W, Hellstrom I, Franke W, Hellstrom KE (1982) Detection of human melanoma-associated antigen, P97, in histological sections of primary human melanoma. Int J Cancer 29: 511–515
Gerweck LE (1985) Hyperthermia in cancer therapy: the biological basis and unresolved questions. Cancer Res 45: 3408–3414
Grassetti DR, Murray JF Jr (1960) Determination of sulfhydryl groups with 2—2″- or 4—4″ dithiodipyridine. Arch Biochem Biophys 119: 41–49
Hellstrom I, Beaumier PL, Hellstom KE (1986) Antitumor effects of L6, an IgG2a antibody that reacts with most human carcinomas. Proc Natl Acad Sci USA 83: 7059–7063
Hellstrom I, Horn D, Linsley P, Brown JP, Brankovan V. Hellstrom KE, (1986) Monoclonal mouse antibodies raised against human lung carcinoma. Cancer 46: 3917–3923
Ishikawa E, Imagawa M, Hashida S, Yoshitaka S, Hamaguci Y, Ueno T, (1983) Enzyme-labeling of antibodies and their fragments for enzyme-immunoassay and immunohistochemical staining. J Immunoassy 4: 209–327
Jahde E, Rajewsky MF (1982) Tumor-selective modification of cellular microenvironment in vivo: effect of glucose infusion on the pH in the normal and malignant rat tissues. Cancer 42: 1505–1512
Lavie E, Beaumier PL, Brodzinski L, Hellstrom KE, Hellstrom I (1989) Evaluation of L6, an anti-carcinoma murine monoclonal antibody, in tumor-bearing nude mice. Radiother Oncol 15: 295–305
Liu F-T, Zinnecker M, Hamaoka T, Katz DH (1979) New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry 18: 690–697
Paschen W, Djuricic B, Miles G, Schmidt-Kastner F, Linn F (1987) Lactate and pH in brain: association and dissociation in different pathophysiological states. J Neurochem 48: 154–159
Rotin D, Wan P, Grinstein S, Tannock I (1987) Cytotoxicity of compounds that interfere with the regulation of intracellular pH: a potential new class of anticancer drugs. Cancer Res 47: 1497–1504
Rowland GF, Axton CA, Baldwin RW, Brown JP, Corvalan JRV, Ebelton MJ, Gore Va, Hellstrom I, Hellstrom KE, Jacobs E, Marsden CH, Pimm MV, Simonds RG, Smith W (1985) Antitumor properties of vindesine-monoclonal antibody conjugates. Cancer Immunol Immunother 19: 1–7
Ryser HJ-P, Shen W-C (1978) Conjugation of poly-l-lysine increases drug transport and overcomes drug resistance in cultured cells. Proc Natl Acad Sci USA 75: 3867–3870
Senter PD, Saulinier MG, Schreiber GJ, Hirschberg DL, Brown JP, Hellstrom I, Hellstrom KE (1988) Antitumor effects of antibody alkaline phosphatase conjugates in combination with etoposide phosphate. Proc Natl Acad Sci USA 85: 4842–4846
Shen W-C, Ryser HJ-P (1978) Conjugation of poly-l-lysine to albumin and horseradish perozidase: a novel method of enhancing the cellular uptake of proteins. Proc Natl Acad Sci USA 75: 1872–1876
Shen W-C, Ryser HJ-P (1981)cis-Aconityl spacer between daunomycin and macromolecular carrier: a model of pH sensitive linkage releasing drug from lysosomotropic conjugate. Biochem Biphys Res Commun 102: 1048–1054
Shen W-C, Yang D, Ryser HJ-P (1984) Colorimetric determination of microgram quantities of polylysine by trypan blue precipitation. Anal Biochem 142: 521–524
Thistlethawaite AJ, Leeper DB, Moylan DJ, Nerlinger RE (1985) pH distribution in human tumors. Int J Radiat Oncol Biol Phys 11: 1647–1652
Thorpe PE, Mason DW, Brown ANF, Simmonds SJ, WC, Cumber AJ, Forrester JA (1982) Selective killing of mallignant cells in leukemic rat bone marrow using an antibody-ricin conjugate. Nature 297: 594
Trouet A, Masquelier M, Baurain R, Deprez-De Campeneere, DA (1982) Covalent linkage between daunorubicin and proteins that is stable in serum and reversible by lysosomal hydrolases, as required for lysosonotropic drug-carrier conjugate: In vitro and in vivo studies. Proc Natl Acad Sci USA 79: 626–629
Tsukada I, Bichof WK, Hibi N, Hirai H, Hurwitz E, Sela M (1982) Effect of conjugates of daunomycin and antibodies to rat alphafetoprotein-producing tumor cells. Proc Natl Acad Sci USA 79: 621–626
Yeh M-Y, Hellstrom I, Hellstrom KE (1981) Clonal variation in expression of human melanoma antigen defined by monoclonal antibody. J Immunol 126: 1312–1317
Zunino F, Savi G, Giuliani F, Gambetta RO, Supino R, Tinelli S, Fezzoni G (1984) Comparison antitumor effects of daunorubicin covalently linked to poly-l-amino acids carriers. Eur J Cancer Clin Oncol 20: 421–425
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lavie, E., Hirschberg, D.L., Schreiber, G. et al. Monoclonal antibody L6-daunomycin conjugates constructed to release free drug at the lower pH of tumor tissue. Cancer Immunol Immunother 33, 223–230 (1991). https://doi.org/10.1007/BF01744941
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01744941