Summary
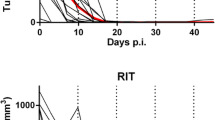
We have used a BALB/c colonic adenocarcinoma (C-26) to evaluate the therapeutic potential of recombinant interleukin-2 (rIL-2) at high and low dosages in combination with or without lymphokine-activated killers (LAK) or tumor-specific, immune lymphocytes in either an adjuvant spontaneous or an artificial metastasis system. Most (≈80%) of the mice that underwent s.c. C-26 tumor excision were shown to die of spontaneous metastasis with lung involvement by 1–4 months after excision. Postsurgical systemic treatment with low-dose rIL-2 (3 × 104 U/day, i.p.) increased the survival rate to 31% as compared to 21% (not significant) in excised controls while administration of high-dose rIL-2 (8 × 104 U/day) led to 53% survival (P <0.01). Both LAK cells and C-26-tumor-immune lymphocytes given during rIL-2 treatment significantly increased the effects of rIL-2 at the low but not at the high-dose, with tumor-immune effectors resulting in the highest percentage (63%) of cures. When mice bearing 3-day artificial lung metastases of C-26 cells were treated with low- or high-dose rIL-2, in combination with or without LAK or tumor-immune lymphocytes, a highly significant reduction or abrogation of the number of lung foci was observed with all treatments, including those involving or tumor-immune lymphocytes alone. Assessment of survival benefit in these mice, however, showed survival prolongation, with 20% cures achieved by low-dose rIL-2 alone and up to 65% cures by LAK in combination with low-dose rIL-2. In this system of artificial metastasis high-dose rIL-2 alone increased the survival time but failed to cure the animals, and the addition of LAK was ineffective whereas that of tumor-immune lymphocytes led to 80% cure. These results suggest that tumorimmune lymphocytes are more effective than LAK when combined with rIL-2 and that caution is necessary in extrapolating findings obtained in artificial metastasis models.
Similar content being viewed by others
References
Brattain MG, Strobel-Stevens J, Five D, Webb M, Sarrif AM (1980) Establishment of mouse colonic carcinoma cell lines with different metastatic properties. Cancer Res 40: 2142
Chou T, Chang AE, Shu S (1988) Generation of therapeutic T lymphocytes from tumor-bearing mice by in vitro sensitization. J Immunol 140: 2453
Colombo MP, Arioli I, Parmiani G (1985) Passive immunotherapy of a BALB/c lymphoma by syngeneic anti-DBA/2 immune lymphoid cells. Characterization of the effector population and evidence for the role of host's non T cells. Cancer Immunol Immunother 20: 198
Fisher B, Packard BS, Read EJ, Carasquillo JA, Carter CS, Topalian SL, Yang JC, Yolles P, Larson SM, Rosenberg SA (1989) Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol 7: 250
Greenberg PD, Kern DE, Cheever MA (1985) Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt1+2− T cells. Tumor eradication does not require participation of cytotoxic T cells. J Exp Med 161: 1122
Greenberg PD (1986) Therapy of murine leukemia with cyclophosphamide and immune Lyt 2+ cells: cytolytic T cells can mediate eradication of disseminated leukemia. J Immunol 136: 1917
Hosokawa M, Yabiku T, Ikeda J, Sawamura Y, Okada F, Komatsumoto M, Tanabe T, Kobayashi H (1988) Effects of a combination of cyclophosphamide and human recombinant interleukin 2 on pulmonary metastasis after the surgical removal of a 3-methylcholanthrene-induced primary tumor in autochthonous mice. Jpn J Cancer Res 79: 1147
Lafreniere R, Rosenberg SA (1985) Adoptive immunotherapy of murine hepatic metastases with lymphokine activated killer cells (LAK) and rIL-2 can mediate the regression of both immunogenic and nonimmunogenic sarcomas and an adenocarcinoma. J Immunol 135: 4273
Lotze MT, Chang AE, Seipp CA, Simpson C, Vetto JT, Rosenberg SA (1986) High dose recombinant interleukin 2 in the treatment of patients with disseminated cancer: responses, treatment-related morbidity, and histologic findings. J Am Med Assoc 256: 3117
Mazumder A, Rosenberg SA (1984) Successful immunotherapy of natural killer-resistant established pulmonary metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by recombinant interleukin 2. J Exp Med 159: 405
Mulé JJ, Ettinghausen SE, Spiess PJ, Shu S, Rosenberg SA (1986) Anti-tumor efficacy of lymphokine-activated killer cells and rIL-2 in vivo: survival benefit and mechanisms of tumor escape in mice undergoing immunotherapy. Cancer Res 46: 676
Mulé JJ, Yang J, Shu S, Rosenberg SA (1986) The antitumor efficacy of lymphokine activated killer cells and recombinant interleukin 2 in vivo: direct correlation between reduction of established metastases and cytolytic activity of lymphokine activated killer cells. J Immunol 136: 3899
Papa MZ, Mulé JJ, Rosenberg SA (1986) Antitumor efficacy of lymphokine-activated killer cells and recombinant interleukin-2 in vivo: successful immunotherapy of established pulmonary metastases from weakly immunogenic and nonimmunogenic murine tumors of three distinct histological types. Cancer Res 46: 4973
Peace DJ, Cheever MA (1989) Toxicity and therapeutic efficacy of high-dose interleukin 2. In vivo infusion of antibody to NK-1.1 attenuates toxicity without compromising efficacy against murine leukemia. J Exp Med 169: 161
Rodolfo M, Parmiani G (1987) Growth inhibition of murine colonic adenocarcinoma by tumor immune but not by rIL-2 activated or alloactivated lymphocytes. Tumori 73: 1
Rodolfo M, Salvi C, Parmiani G (1989) Influence of the donors' clinical status on in vitro and in vivo tumor cytotoxic activation of interleukin 2 exposed lymphocytes and their circulation in different organs. Cancer Immunol Immunother 28: 136
Rosenberg SA (1988) Immunotherapy of cancer using interleukin 2. Immunol Today 9: 58
Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE (1988) Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N Engl J Med 319: 1676
Salup RR, Wiltrout RH (1986) Adjuvant immunotherapy of established murine renal cancer by interleukin 2-stimulated cytotoxic lymphocytes. Cancer Res 46: 3358
Schoof DD, Gramolini BA, Davidson DL, Massaro AF, Wilson RE, Eberlein TJ (1988) Adoptive immunotherapy of human cancer using low-dose recocmbinant interleukin 2 and lymphokine-activated killer cells. Cancer Res 48: 5007
Spiess PJ, Yang JC, Rosenberg SA (1987) In vivo antitumor activity of tumor infiltrating lymphocytes expanded in recombinant interleukin-2. J Natl Cancer Inst 79: 1067
Talmadge JE, Phillips H, Schindler J, Tribble H, Pennington R (1987) Systematic preclinical study on the therapeutic properties of recombinant human interleukin 2 for the treatment of metastatic disease. Cancer Res 47: 5725
Thompson JA, Peace DJ, Klarnet JP, Kern DE, Greenberg PD, Cheever MA (1986) Eradication of disseminated murine leukemia by treatment with high-dose interleukin 2. J Immunol 137: 3675
Treves AJ, Cohen IR, Feldmann M (1975) Immunotherapy of lethal metastases by lymphocytes sensitized against tumor cells in vitro. J Natl Cancer Inst 54: 777
Vaage J (1987) Local and systemic effects during interleukin-2 therapy of mouse mammary tumors. Cancer Res 47: 4296
Wang J, Walle A, Gordon B, Novogrodsky A, Suthanthiran M, Rubin A, Morrison H, Silver RT, Stenzel KH (1987) Adoptive immunotherapy for stage IV renal cell carcinoma: a novel protocol utilizing periodate and interleukin-2-activated autologous leukocytes and continous infusions of low-dose interleukin-2. Am J Med 83: 1016
West WH, Tauer KW, Yannelli JR, Marshakk JD, Orr DW, Thurmann GB, Oldham RK (1987) Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med 316: 898
Wexler H (1966) Accurate identification of experimental pulmonary metastasis. J Natl Cancer Inst 36: 641
Wiebke EA, Rosenberg JA, Lotze MT (1988) Acute immunologic effects of interleukin-2 therapy in cancer patients: decreased delayed hypersensitivity response and decreased proliferative response to soluble antigens. J Clin Oncol 6: 1440
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodolfo, M., Salvi, C., Bassi, C. et al. Adoptive immunotherapy of a mouse colon carcinoma with recombinant interleukin-2 alone or combined with lymphokine-activated killer cells or tumor-immune lymphocytes. Cancer Immunol Immunother 31, 28–36 (1990). https://doi.org/10.1007/BF01742492
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01742492