Summary
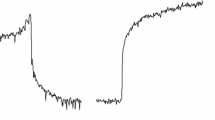
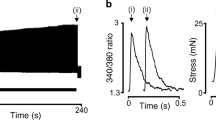
Rapid stretching of smooth muscle induces a large increase in stress (resistance to stretch), and stress gradually decreases to intermediate values (stress relaxation). This study elucidated whether [Ca2+]-dependent crossbridge activation or passive mechanical structures were responsible for resistance to stretch and stress relaxation in swine carotid media, a tissue that does not exhibit a myogenic repsonse. Tissues were equilibrated at the optimal length for stress development (L0) and loaded with aequorin to estimate myoplasmic [Ca2+]. In tissues activated with contractile agonists, both resistance to stretch and the rate of stress relaxation appeared to correlate best with the stress present before stretch. Stretch-induced [Ca2+] transients had no major role in determining resistance to stretch or stress relaxation. In the unstimulated swine carotid, resistance to stretch was only slightly reduced and stress relaxation not affected by removal of extracellular Ca2+, suggesting that resistance to stretch and the rate of stress relaxation in unstimulated tissues was predominantly dependent on the passive components of smooth muscle rather than attached, Ca2+-dependent crossbridges. Incubation of tissues with tetraethylammonium ion had no measurable effect on stretch-induced [Ca2+] transients but increased resistance to stretch, suggesting that stretch-induced myogenic contractions may be mediated by an increase in the sensitivity of the contractile apparatus to [Ca2+]. Despite the ability of stretch to produce substantial increases in myoplasmic [Ca2+], the contractile apparatus of swine carotid is quite insensitive to the stretch-induced [Ca2+] elevations.
Similar content being viewed by others
References
Alexander, R. S. (1957) Elasticity of muscular organs. InTissue Elasticity (edited byRemington, J. W.), pp. 111–22. Washington DC: American Physiological Society.
Allen, D. G. &Blinks, J. R. (1979) The interpretation of light signals from aequorin-injected skeletal and cardiac muscle cells: a new method of calibration. InDetection and Measurements of Free Ca 2+ in Cells (edited byAshley, C. C. &Campbell, A. K.), pp. 159–74. Amsterdam: Elsevier/ North-Holland Biomédical Press.
Barany, K., Ledvora, R. F., Mougios, V. &Barany, M. (1985) Stretch-induced myosin light chain phosphorylation and stretch-release-induced tension development in arterial smooth muscle.J. Biol. Chem. 260, 7126–30.
Bozler, E. (1957) Extensibility of contractile elements. InTissue Elasticity (edited byRemington, J. W.), pp. 102–10. Washington DC: American Physiological Society.
Driska, S. P., Damon, D. N. &Murphy, R. A. (1978) Estimates of cellular mechanics in an arterial smooth muscle.Biophys. J. 24, 525–40.
Driska, S. P., Aksoy, M. O. &Murphy, R. A. (1981) Myosin light chain phosphorylation associated with contraction in arterial smooth muscle.Am. J. Physiol. 240, C222-C233.
Gilbert, E. K., Weaver, B. A. &Rembold, C. M. (1991) Depolarization decreases the [Ca2+]i sensitivity of myosin light chain kinase in arterial smooth muscle: a comparison of aequorin and fura 2 [Ca2+] estimates.FASEB J. 5 (in press).
Hai, C-M. &Murphy, R. A. (1989) Ca2+, crossbridge phosphorylation, and contraction.Ann. Rev. Physiol. 51, 285–98.
Herlihy, J. T. &Murphy, R. A. (1973) Length-tension relationship of smooth muscle of the hog carotid artery.Circ. Res. 33, 275–83.
Himpens, B. &Somlyo, A. P. (1988) Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea pig smooth muscle.J. Physiol. 395, 507–30.
Himpens, B., Matthijs, G., Somlyo, A. V., Butler, T. M. &Somlyo, A. P. (1988) Cytoplasmic free calcium, myosin light chain phosphorylation, and force in phasic and tonic smooth muscle.J. Gen. Physiol. 92, 713–29.
Kirber, M. T., Walsh, J. V., Jr. &Singer, J. J. (1988) Stretch-activated ion channels in smooth muscle: a mechanisms for the initiation of stretch-induced contraction.Pflügers Arch. 412, 339–45.
Laher, I. &Bevan, J. A. (1989) Staurosporine, a protein kinase C inhibitor, attenuates Ca2+-dependent stretch-induced vascular tone.Biochem. Biophys. Res. Commun. 158, 58–62.
Laher, I., Vorkapic, P., Dowd, A. L. &Bevan, J. A. (1989) Protein kinase C potentiates stretch-induced cerebral artery tone by increasing intracellular sensitivity to Ca2+.Biochem. Biophys. Res. Commun. 165, 312–18.
Lansman, J. B., Hallam, T. J. &Rink, T. J. (1987) Single stretch-activated ion channels in vascular cells as mechano-receptors.Nature 325, 811–13.
Morgan, J. P. &Morgan, K. G. (1984) Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein.J. Physiol. (Lond.)351, 155–67.
Murphy, R. A. (1980) Mechanics of vascular smooth muscle. InHandbook of Physiology, The Cardiovascular System, Vol. II.Vascular Smooth Muscle (edited byBohr, D. F., Somlyo, A. P. &Sparks, H. V.), pp. 325–51. Bethesda: American Physiological Society.
Rembold, C. M. (1989) Desensitization of swine arterial smooth muscle to transplasmalemmal Ca2+ influx.J. Physiol. (Lond.)416, 273–90.
Rembold, C. M. (1990) Modulation of the [Ca2+]-sensitivity of myosin phosphorylation in intact swine arterial smooth muscle.J. Physiol. (Lond.)429, 77–94.
Rembold, C. M. &Murphy, R. A. (1986) Myoplasmic calcium, myosin phosphorylation, and regulation of the cross-bridge cycle in swine arterial smooth muscle.Circ. Res. 58, 803–15.
Rembold, C. M. &Murphy, R. A. (1988) Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle.Circ. Res. 63, 593–603.
Rembold, C. M. &Murphy, R. A. (1989) Histamine concentration and Ca2+ mobilization in arterial smooth muscle.Am. J. Physiol. Cell Physiol. 257 (Cell Phys 26), C122-C128.
Rembold, C. M. &Murphy, R. A. (1990a) Muscle length, shortening, myoplasmic [Ca2+], and activation of arterial smooth muscle.Circ. Res. 66, 1354–61.
Rembold, C. M. &Murphy, R. A. (1990b) The latchbridge model in smooth muscle: [Ca2+] can quantitatively predict stress.Am. J. Physiol. Cell Physiol. 259, C251-C257.
Siegman, M. J., Butler, T. M., Mooers, S. U. &Davies, R. E. (1976) Calcium-dependent resistance to stretch and stress relaxation in resting smooth muscles.Am. J. Physiol. 231, 1501–8.
Smith, S. J. &Augustine, G. J. (1988) Calcium ions, active zones and synaptic transmitter release.TINS 11, 458–64.
Stephens, N. L., Kroeger, E. A. &Kromer, U. (1975) Induction of a myogenic response in tonic airway smooth muscle by tetraethylammonium.Am. J. Physiol. 228, 628–32.
Stull, J. T., Hsu, L-C., Tansey, M. G. &Kamm, K. E. (1990) Myosin light chain kinase phosphorylation in tracheal smooth muscle.J. Biol. Chem. 265, 16683–90.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rembold, C.M. Resistance to stretch, [Ca2+]i, and activation of swine arterial smooth muscle. J Muscle Res Cell Motil 13, 27–34 (1992). https://doi.org/10.1007/BF01738424
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01738424