Summary
Cytochromec oxidase from the inner membrane of yeast mitochondria consists of seven nonidentical protein subunits, three being synthesized on mitochondrial ribosomes (molecular weights I: 43 K, II: 34 K, and III: 24 K) and four being made on cytoplasmic ribosomes (molecular weights IV: 14 K, V: 12 K, VI: 12 K, and VII: 4.5 K).
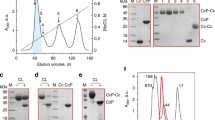
In the present study all four cytoplasmically synthesized subunits of the enzyme were isolated on a large scale using ion exchange chromatography and gel filtartion. Their amino acid composition as well as their amino- and carboxy-terminal amino acid residues have been determined. Sequence determinations of sub-units IV and VI are already in an advanced state. The sequence of subunit VI is characterized by a large amino-terminal stretch dominated by charged amino acid residues followed by a cluster of hydrophobic amino acids.
The binding site of yeast cytochrome oxidase for cytochromec was studied by chemical crosslinking experiments. The formation of a disulfide bridge between the two proteins was observed by using cytochromec from yeast modified with 5-thionitrobenzoate at the cysteinyl residue in position 107. Alternatively, a disulfide between yeast cytochromec and the oxidase could be formed directly by oxidation with copper phenanthroline. Gel electrophoresis of the crosslinked complexes in sodium dodecyl sulfate revealed a new protein band with an apparent molecular weight of 38 K. This new band appears to be derived from cytochromec and from subunit III of cytochrome oxidase.
Similar content being viewed by others
References
Schatz, G. and Mason, T. L., 1974. Annu. Rev. Biochem. 43, 51–87.
Mason, T. L. and Schatz, G., 1973. J. Biol. Chem. 248, 1355–1360.
Rubin, M. S. and Tzagoloff, A., 1973. J. Biol. Chem. 248, 4275–4279.
Tzagoloff, A. and Meagher, P., 1972. J. Biol. Chem. 247, 594–603.
Weiss, H. and Ziganke, B., 1974. Eur. J. Biochem. 41, 63–71 (74).
Mason, T. L., Poyton, R. O., Wharton, D. C. and Schatz, G., 1973. J. Biol. Chem. 248, 1346–1354.
Rubin, M. S. and Tzagoloff, A., 1973. J. Biol. Chem. 248, 4269–4274.
Poyton, R. O. and Schatz, G., 1974. J. Biol. Chem. 250, 752–761.
Eytan, G. D. and Schatz, G., 1975. J. Biol. Chem. 250, 767–774.
Deters, D. E., Müller, U. and Homberger, H., 1975. Anal. Biochem., in press.
Ellman, G. L., 1959. Arch. Biochem. Biophys. 82, 70–77.
Birchmeier, W., Wilson, K. J. and Christen, P., 1973. J. Biol. Chem. 248, 1751–1755.
Wang, K. and Richards, F. M., 1974. J. Biol. Chem. 249, 8005–8018.
Capaldi, R. A. and Vanderkooi, G., 1972. Proc. Natl. Acad. Sci. USA 69, 903–932.
Narita, K., Titani, K., Yaoi, Y., Murakami, H., Kimura, M. and Vanecek, J., 1936. Biochim. Biophys. Acta 73, 670–673.
Narita, K., Titani, K., Yaoi, Y. and Murakami, H., 1963. Biochim. Biophys. Acta 77, 688–690.
Dickerson, R. E., Takano, T., Eisenberg, D., Kallai, O. B., Samson, L., Cooper, A. and Margoliash, E., 1971. J. Biol. Chem. 246, 1511–1535.
Dickerson, R. E.: Scientific American, p. 58–70 (April 1972).
Eytan, G., Caroll, R. C., Schatz, G. and Racker, E., 1976. J. Biol. Chem., in press.
Author information
Authors and Affiliations
Additional information
Recipient of a fellowship from the Swiss National Science Foundation. Present address: Department of Biology, University of California at San Diego, La Jolla, Calif. 92037 (USA).
Rights and permissions
About this article
Cite this article
Birchmeier, W. Structure of cytochromec oxidase from baker's yeast—a progress report. Preparation of four subunits for amino acid sequence determination and attempts to localize the cytochromec binding site. Mol Cell Biochem 14, 81–86 (1977). https://doi.org/10.1007/BF01734168
Issue Date:
DOI: https://doi.org/10.1007/BF01734168