Summary
-
1.
The regulatory properties of two interconvertible kinetic forms of class A pyruvate kinase from Ehrlich ascites tumor cells have been studied with a partially purified enzyme preparation free of interfering enzymatic activities.
-
2.
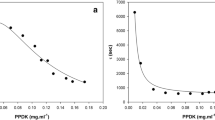
The hyperbolic form shows Michaelis-Menten kinetics for P-pyruvate, with high affinity for this substrate and low affinity for the inhibitory amino acids alanine and phenylalanine. The sigmoidal form displays positive cooperativity respect to P-pyruvate (n = 1.4), with lower affinity for this substrate and higher affinity for the inhibitory amino acids.
-
3.
The equilibrium between the hyperbolic and the sigmoidal forms of the enzyme is affected by substrates and effectors. P-pyruvate, ADP and Fru-P2 shift the equilibrium to the hyperbolic form while ATP, alanine and phenylalanine stabilize the sigmoidal form.
-
4.
Effector metabolites affect the molecular weight of the protein, acting on an equilibrium between dimers and tetramers. P-pyruvate and ADP associate the enzyme to a tetramer while ATP, alanine and phenylalanine favor the occurrence as a dimer. The positive modifier Fru-P2 did not associate the enzyme to the tetramer, even at 1mm concentration.
-
5.
A tentative molecular model for pyruvate kinase A on the basis of the kinetic and aggregation interconversion is proposed.
Similar content being viewed by others
Abbreviations
- DTE:
-
dithioerythritol
- Fru-P2 :
-
fructose 1,6-bis-phosphate
- P-pyruvate:
-
phosphoenolpyruvate
References
Susor, W. A. and Rutter, W. J., 1968. Biochem. Biophys. Res. Commun. 30, 14–20.
Imamura, K. and Tanaka, T., 1972. J. Biochem. Tokyo 71, 1043–1051.
Imamura, K., Taniuchi, K. and Tanaka, T., 1972. J. Biochem. Tokyo 72, 1001–1015.
Carbonell, J., Felíu, J. E., Marco, R. and Sols, A., 1973. Eur. J. Biochem. 37, 148–156.
Pogson, C. I., 1968. Biochem. J. 110, 67–77.
Marco, R., Carbonell, J. and Llorente, P., 1971. Biochem. Biophys. Res. Commun. 43, 126–132.
Walker, P. R. and Potter, V. R., 1973. J. Biol. Chem. 248, 4610–4616.
Ibsen, K. H. and Kruger, E., 1973. Arch. Biochem. Biophys. 157, 509–513.
Felíu, J. E., Díaz-Gil, J. J., García-Cañero, R. and Gosálvez, M., 1975. FEBS Lett. 50, 334–338.
Sillero, A., Sillero, M. A. G. and Sols, A., 1969. Eur. J. Biochem. 10, 351–354.
Adams, H., 1963., in Bergmeyer, H. U. (Ed.) Methods of Enzymatic Analysis, Academic Press, New York, p. 573–577.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J., 1951. J. Biol. Chem. 193, 265–272.
Altman, P. L. and Dittmer, D. S., 1973. Biology Data Book, 2nd edn, Fed. Amer. Soc. Exptl. Biol., Washington.
Barman, T. E., 1969. Enzyme Handbook, Springer-Verlag, Berlin-New York.
Imamura, K. and Tanaka, T., 1972. J. Biochem. Tokyo 71, 1043–1051.
Rozengurt, E., Jiménez de Asúa, L. and Carminatti, H., 1969. J. Biol. Chem. 244, 3142–3147.
Frieden, C., 1970. J. Biol. Chem. 245, 5788–5799.
Koshland, D. E., Jr., 1970. in Boyer, P. D. (Ed.) The Enzymes, Academic Press, New York, vol. 1, p. 341–396.
Morrison, J. F. and Heyde, E., 1972. An. Rev. Biochem. 41, 29–54.
Sparmann, G., Schulz, J. and Hofman, E., 1973. FEBS Lett. 36, 305–308.
Schulz, J., Sparmann, G. and Hofman, E., 1975. FEBS Lett. 50, 346–350.
Reynard, A. M., Hass, L. F., Jacobsen, D. D. and Boyer, P. D., 1961. J. Biol. Chem. 236, 2277–2283.
Hess, B. and Kutzbach, C., 1971. Hoppe-Seyler's Z. Physiol. Chem. 352, 453–458.
Ibsen, H. K. and Trippet, P., 1971. Life Sci. 10, 1021–1029.
Ibsen, H. K. and Trippet, P., 1972. Biochemistry 11, 4442–4450.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Felíu, J.E., Sols, A. Interconversion phenomena between two kinetic forms of class a pyruvate kinase from Ehrlich ascites tumor cells. Mol Cell Biochem 13, 31–44 (1976). https://doi.org/10.1007/BF01732393
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01732393