Summary
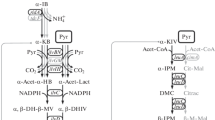
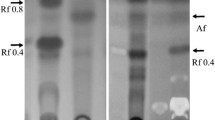
DL-phenylalanine hydroxamate inhibited the growth ofBacillus subtilis NCTC 8533 and this inhibition was most effectively reversed by L-phenylalanine. Mutants ofB. subtilis, resistant to the analogue, excreted L-phenylalanine into the medium in significant amounts. The accumulation of amino acid was further increased after mutation of analogue-resistant strains to a requirement for tyrosine. Strain THrP-17 was resistant to DL-phenylalanine hydroxamate and had an absolute requirement for tyrosine and accumulated 1.37 g L-phenylalanine per litre of medium.
Similar content being viewed by others
References
F. Gibson and J. Pittard. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol. Rev. 32, 465–492 (1968).
O. Karlstrom, Methods for production of mutants suitable as amino acid fermentation organisms. Biotechnol. Bioeng. 7, 245–268 (1965).
E. A. Adelberg. Selection of bacterial mutants which excrete antagonists of antimetabolites, J. Bacteriol. 76, 326 (1958).
T. Asai. General aspects of amino acid fermentations in Japan. Recent Prog. Microbiol. 8, 325–333 (1963).
E. L. Dulaney, Industrial implications of microbiological production of amino acids. Recent Prog. Microbiol. 8, 317–324 (1963).
K. Nakayama, S. Kitada, Z. Sato and S. Kinoshita. Induction of nutritional mutants of glutamic acid bacteria and their amino acid accumulation. J. Gen. Appl. Microbiol. 7, 41–51 (1961).
B. D. Davis and E. S. Mingioli. Mutants of E. coli requiring methionine and vitamin B. J. Bacteriol. 60, 17–28 (1950).
T. Tosa and L. I. Pizer. Effect of serine hydroxamate on the growth of Escherichia coli. J. Bacteriol. 106, 966–971 (1971).
T. Tosa and L. I. Pizer. Biochemical bases for the antimetabolite action of L-serine hydroxamate. J. Bacteriol. 106, 972–982 (1971).
M. Kisumi, S. Komatsubara, M. Sugiura and I. Chibata. Isoleucine hydroxamate, an isoleucine antagonist. J. Bacteriol. 107, 741–745 (1971).
M. Kisumi, J. Kato, M. Sugiura and I. Chibata. Production of L-arginine by arginine hydroxamateresistant mutants of Bacillus subtilis. Appl. Microbiol. 22, 987–991 (1971).
L. Fowden, D. Lewis and H. Tristram. Toxic acids: their action as antimetabolistes. Adv. Enzymol. 29, 89–163 (1968).
S. Alikhanian, Applied aspects of microbial genetics. Current Topics Microbiol. Immunol. 53, 91–148 (1970).
H. Hagino and K. Nakayama. L-tyrosine production by analog-resistant mutants derived from a phenylalanine auxotroph of Corynebacterium glutamicum. Agr. Biol. Chem. 37, 2013–2023 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McEvoy, J.J., Joyce, A. Production of L-phenylalanine by DL-phenylalanine hydroxamate-resistant tyr− mutants of Bacillus subtilis. Mol Cell Biochem 4, 191–195 (1974). https://doi.org/10.1007/BF01731480
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01731480