Summary
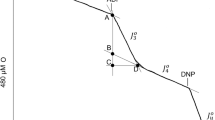
The extent of the deactivation of the mitochondrial succinate dehydrogenase by oxaloacetate is a function of the redox state of the enzyme. Oxidized enzyme is deactivated by much lower concentrations of oxaloacetate than those needed to deactivate reduced enzyme. An accurate method for measuring this relationship is the redox titration of the enzymic activity of succinate dehydrogenase, carried out in the presence of oxaloacetate. For each concentration of oxaloacetate a different redox titration curve was recorded with the apparent mid-potential decreasing with increasing oxaloacetate. These results are compatible with a model which proposes that both oxidized and reduced enzymes can form the catalytically non-active complex with oxaloacetate, but that the complex formed by the oxidized enzyme is more stable than that formed by the reduced enzyme. When the oxaloacetate concentration is low, reduction of the enzyme will lower the fraction of the succinate dehydrogenase-oxaloacetate complex, a reaction which we observe as reductive activation of the enzyme. If this experiment is repeated in the presence of high concentration of oxaloacetate, no activation of the enzyme takes place, but the low stability of the reduced enzyme oxaloacetate complex is revealed by the rapid exchange of the enzyme-bound oxaloacetate with the free ligand. The rate of this exchange is extremely slow at high positive potential and becomes faster upon lowering of the poise potential.
The reductive activation of the succinate dehydrogenase is regarded as a two step reaction. In the first step the reduced non-active complex releases the oxaloacetate and in the second step the active form of the enzyme is evolved. These two steps can be observed experimentally; Reductive activation at a redox potential higher than the mid-potential of the oxaloacetate-malate couple (− 166 mV) is characterized by Ea = 18 Kcal/mole, the final equilibrium level of activation decreases upon lowering of the temperature. Reductive activation of the enzyme at −240 mV is a very rapid reaction which goes to completion at all temperatures tested and has an activation energy of 12.5 Kcal/mole. The mechanism of the reductive activation and its possible role in the regulation of succinate dehydrogenase in the mitochondria is discussed.
Similar content being viewed by others
References
M. Gutman, E. B. Kearney and T. P. Singer, Biochemistry 10, 2726–2733 (1971).
E. B. Kearney, J. Biol. Chem. 229, 363–375 (1957).
A. D. M. Klaasse and E. C. Slater, Z. Naturforch 27b, 1077–1078 (1972).
J. Salach and T. P. Singer, J. Biol. Chem. 249, 3765–3767 (1944).
E. B. Kearney, B. A. C. Ackrell, M. Mayer and T. P. Singer, J. Biol. Chem. 249, 2016–2020 (1974).
B. A. C. Ackrell, E. B. Kearney and M. Mayer, J. Biol. Chem. 249, 2021–2027 (1974).
M. Gutman and N. Silman, Mol. and cellular Biochem. 7. 1, 51–58 (1975).
L. Szarkowska, Arch. Biochem. Biophys. 113, 519–525 (1966).
D. V. Dervartanian and C. Veeger, Biochem. Biophys. Acta 92, 233–247 (1964).
A. Kroger and M. Klingenberg, Eur. J. Biochem. 34, 313–323 (1973).
K. Burton and T. M. Wilson, Biochem. J. 54, 86–94 (1953).
T. P. Singer, E. B. Kearney and W. C. Kenney, Advan. Enzymol 37, 189–272 (1973).
J. R. Williamson, C. M. Smith, K. F. La Noue and J. Bryla, in Energy Metabolism and Regulation of Metabolic Processes in Mitochondria. Mehlman, M. A. and Hanson, R. W., Eds., Academic Press, New York, p. 185 (1972).
M. Gutman, E. B. Kearney and T. P. Singer, Biochemistry 10, 4763–4769 (1971).
M. Gutfreund and E. A. Jones, Biochem. J. 90, 208–213 (1964).
A. Kroger and M. Klingenberg, Biochem. Z. 344, 317–336 (1966).
Author information
Authors and Affiliations
Additional information
This publication is dedicated to the memory of my friend and colleague Mrs. NitzaSilman-Movshovitz, who died before the submission of the manuscript.
Invited contribution.
Rights and permissions
About this article
Cite this article
Gutman, M., Silman, N. The steady state activity of succinate dehydrogenase in the presence of opposing effectors. Mol Cell Biochem 7, 177–185 (1975). https://doi.org/10.1007/BF01731407
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01731407