Abstract
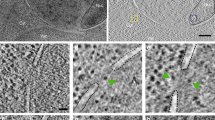
The structural features of the yeast DNA-dependent RNA polymerase A (I) were examined by Scanning Transmission Electron Microscopy. The enzyme was adsorbed in its monomeric form and negatively stained prior to digital image acquisition at low dose. The signal to noise ratio of single particle images was improved through averaging of a large number of previously aligned and partitioned images. Six classes of images were obtained reproducibly which corresponded to different projections of the enzyme. The enzyme structure was characterized by its elongated shape of 15.5 by 10.5 nm and by the presence of two curved arms which defined a longitudinal cleft. By analogy with theEscherichia coli enzyme, these arms could correspond to the two large subunits A135 and A190.
Similar content being viewed by others
References
Ahearn JM, Bartolomei MS, West ML, Cisek LJ, Corden JL (1987) Cloning and sequence analysis of the mouse genomic locus encoding the largest subunit of RNA polymerase II. J Biol chem 262:10695–10705
Allison LA, Moyle M, Shales M, Ingles CJ (1985) Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell 42:599–610
Buhler J-M, Huet J, Davies KE, Sentenac A, Fromageot P (1980) Immunological studies of yeast nuclear RNA polymerases at the subunit level. J Biol Chem 255:9949–9954
Bull P, Garrido J (1982) Structure of yeast RNA polymerases determined by electron microscopy. Arch Biochem Biophys 219:163–166
Darst SA, Ribi HO, Pierce DW, Kornberg RD (1988) Two-dimensional crystals ofEscherichia coli RNA polymerase holoenzyme on positively charged lipid layers. J Mol Biol 203:269–273
Darst SA, Kubalec EW, Kornberg RD (1989) Three-dimensional structure ofEscherichia coli RNA polymerase holoenzyme determined by electron crystallography. Nature 340:730–732
Falkenburg D, Dworniczak B, Faust DM, Bautz EKF (1987) RNA polymerase II ofDrosophila. Relation of its 140 000 Mr subunit to the beta subunit ofEscherichia coli RNA polymerase. J Mol Biol 195:929–937
Frank J, Goldfarb W, Eisenberg D, Baker TS (1978) Reconstitution of glutamine synthetase using computer averaging. Ultramicroscopy 3:283–290
Frank J, Verschoor A, Boublick M (1982) Multivariate statistical analysis of ribosome electron micrographs. L and R lateral views of the 40S subunit from HeLa cells. J Mol Biol 161:107–137
Grachev MA, Mustaev AA, Zaychikov EF, Lindner AJ, Hartmann GR (1989a) Localization of the binding site for the initiating substrate on the RNA polymerase fromSulfolobus acidocaldarius. FEBS Lett 250:317–322
Grachev MA, Lukhtanov EA, Mustaev AA, Zaychikov EF, Abdukayumov MN, Rabinov IV, Richter VI, Skoblov YS, Chistyakov PG (1989b) Studies of the functional topology ofEscherichia coli RNA polymerase. A method for localization of the sites of affinity labelling. Eur J Biochem 180:577–585
Hillel Z, Wu C-W (1977) Subunit topology of RNA polymerase fromEscherichia coli. A cross-linking study with bifunctional reagents. Biochemistry 16:3334–3342
Himmelfarb HJ, Simpson EM, Friesen JD (1987) Isolation and characterization of temperature-sensitive RNA polymerase II mutants ofSaccharomyces cerevisiae. Mol Cell Biol 7:2155–2164
Homo JC (1980) Micro-cryostat for high resolution electron microscope specimen stage. Electron Microsc 1:92
Huet J, Buhler J-M, Sentenac A, Fromageot P (1975) Dissociation of two polypeptide chains from yeast RNA polymerase A. Proc Natl Acad Sci USA 72:3034–3038
Huet J, Sentenac A, Fromageot P (1982) Spot-immunodetection of conserved determinants in eukaryotic RNA polymerases. J Biol Chem 257:2613–2618
Jokerst RS, Weeks JR, Zehring WA, Greenleaf Al (1989) Analysis of the gene encoding the largest subunit of RNA polymerase II inDrosophila. Mol Gen Genet 215:266–275
Kownin P, Bateman E, Paule MR (1987) Eukaryotic RNA polymerase I promoter binding is directed by protein contacts with transcription initiation factor and is DNA sequence-independent. Cell 50:693–699
Lewis MK, Burgess AA (1982) Eukaryotic RNA polymerases. In Boyer PD (Escherichia coli) (ed) The enzymes, vol 15. Academic Press, New York
Mann C, Buhler J-M, Treich I, Sentenac A (1987) RPC40, a unique gene for a subunit shared between yeast RNA polymerases A and C. Cell 48:627–637
Meisenberger O, Piltz I, Heumann H (1980) Small angle X-ray study of DNA-dependent RNA polymerase subunit alpha-2 fromEscherichia coli. FEBS Lett 122:117–120
Mémet S, Saurin W, Sentenac A (1988a) RNA polymerases B and C are more closely related to each other than RNA polymerase A. J Biol Chem 263:10048–10051
Mémet S, Gouy M, Marck C, Sentenac A, Buhler J-M (1988b) RPA190, the gene coding for the largest subunit of yeast RNA polymerase A. J Biol Chem 263:2830–2839
Saxton WO (1980) Digital processing of electron images — A survey of motivations and methods. Proc 7th Eur Cong Electron Microscopy: The Hague, vol 2, pp 682–689
Schultz P, Weiss E, Colin P, Régnier E, Oudet P (1986) Characterization of SV40 chromatin by mass determination of STEM. Chromosoma 94:189–198
Sentenac A (1985) Eukaryotic RNA polymerases. CRC Critical Rev Biochemistry 31–90
Sentenac A, Hall BD (1982) Yeast nuclear RNA polymerases and their role in transcription. In: Strathern JN, Jones EW, Broach JR (eds) The molecular biology of the yeastSaccharomyces cerevisae: Metabolism und gene expression. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 561–606
Shaner SL, Piatt DM, Wensley CG, Yu H, Burgess RP, Record MT (1982) Aggreation equilibria ofEscherichia coli RNA polymerase: Evidence for anion-linked conformational transitions in the promoters of core and holoenzyme. Biochemistry 21:5539–5551
Stöckel P, May R, Strell A, Cejka Z, Hoppe W, Heumann H, Zillig W, Crespi HL (1980) The core subunit structure in RNA polymerase holoenzyme determined by neutron small-angle scattering. Eur J Biochem 112:419–423
Sweetzer D, Nonet M, Joung R (1987) Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci USA 84:1192–1196
Tichelaar W, Stender W, Van Bruggen EFJ (1980) In: Brederoo P, De Priester W (eds) Electron microscopy. vol 2. Seventh European Congress on Electron Microscope Foundation, Leiden, pp 590–591
Tichelaar W, Schutter WG, Arnberg AC, Van Bruggen EFJ, Stender W (1983) The quaternary structure ofEscherichia coli RNA polymerase studied with (scanning) transmission (immuno)electron microscopy. Eur J Biochem 135:263–269
Tichelaar W, Schutter WG, Wichertjes T, Van Bruggen EFJ (1984) The monomeric arrangement in the dimer ofEscherichia coli RNA polymerase holoenzyme studied with scanning transmission electron microscopy. Micron Microsc Acta 15:195–203
Van Heel M (1989) Classification of very large electron microscopical image data sets. Optik 82:114–126
Van Heel M, Frank J (1981) Use of multivariate statistics in analysing the images of biological macromolecules. Ultramicroscopy 6:187–194
Van Heel M, Keegstra W (1981) IMAGIC: A fast, flexible and friendly image analysis software system. Ultramicroscopy 7:113–130
Wagenknecht T, Frank J, Boublik M, Nurse K, Ofengand J (1988) Direct localization of the tRNA-anticodon interaction site on theEscherichia coli 30S ribosomal subunits by electron microscopy and computerized image averaging. J Mol Biol 203:753–760
Wilson DW, Meacock PA (1988) Extranuclear gene expression in yeast: evidence for a plasmid-encoded RNA polymerase of unique structure. Nucleic Acids Res 16:8097–8112
Wittekind M, Dodd J, Vu L, Kolb JM, Buhler J-M, Sentenac A, Nomura M (1988) Isolation and characterization of temperature-sensitive mutation in RPA190, the gene encoding the largest subunit of RNA polymerase I fromSaccharomyces cerevisiae. Mol Cell Genet 8:3997–4008
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schultz, P., Nobelis, P., Colin, P. et al. Electron microscopic study of yeast RNA polymerase A: Analysis of single molecular images. Chromosoma 99, 196–204 (1990). https://doi.org/10.1007/BF01731130
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01731130