Abstract
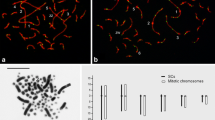
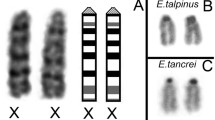
Differences in length of the heterochromatic short arms of the X and Y chromosomes in individuals ofPeromyscus beatae are hypothesized to result from unequal crossing over. To test this hypothesis, we examined patterns of synapsis, chiasma formation, and segregation for maleP. beatae which were either heterozygous or homozygous for the amount of short-arm sex heterochromatin. Synaptonemal complex analysis demonstrated that mitotic differences in heterochromatic shortarm lengths between the X and Y chromosomes were reflected in early pachynema as corresponding differences in axial element lengths within the pairing region of the sex bivalent. These length differences were subsequently eliminated by synaptic adjustment such that by late pachynema, the synaptonemal complex configurations of the XY bivalent of heterozygotes were not differentiable from those of homozygotes. Crossing over between the heterochromatic short arms of the XY bivalent was documented by the routine appearance of a single chiasma in this region during diakinesis/metaphase I. Sex heterochromatin heterozygotes were characterized by the presence of asymmetrical chiasma between the X and Y short arms at diakinesis/metaphase I and sex chromosomes with unequal chromatid lengths at metaphase II. These data corroborate our hypothesis on the role of unequal crossing over in the production and propagation of X and Y heterochromatin variation and suggest that, in some cases, crossing over can occur during the process of synaptic adjustment.
Similar content being viewed by others
References
Allen JW (1979) BrdU-dye characterization of late replication and meiotic recombination in Armenian hamster germ cells. Chromosoma 74:189–207
Arrighi FE, Hsu TC, Pathak S, Sawada H (1974) The sex chromosomes of the Chinese hamster: Constitutive heterochromatin deficient in repetitive DNA sequences. Cytogenet Cell Genet 13:268–274
Baker RJ, Haiduk MW, Robbins LW, Cadena A, Koop BF (1982) Chromosomal studies of South American bats and their systematic implications. In: Mare MA, Genoways HH (eds) Mammalian biology in South America. University of Pittsburgh Press, Pittsburgh, pp 303–327
Baverstock PR, Watts CHS, Hogarth JT (1977) Polymorphism of the X-chromosome, Y-chromosome and autosomes in the Australian hopping mice,Notomys alexis, N. cervinus andN. fuscus (Rodentia, Muridae). Chromosoma 61:234–256
Buckle V, Mondello C, Darling S, Craig IW, Goodfellow PN (1985) Homologous expressed genes in the human sex chromosome pairing region. Nature 317:739–741
Burgos M, Jiménez R, Olmos DM, Diaz de la Guardia R (1988) Heterogeneous heterochromatin and size variation in the sex chromosomes ofMicrotus cabrerae. Cytogenet Cell Genet 47:75–79
Burgoyne PS (1982) Genetic homology and crossing over in the X and Y chromosomes of mammals. Hum Genet 61:85–90
Chandley AC, Goetz P, Hargreave TB, Joseph AM, Speed RM (1984) On the nature and extent of XY pairing at meiotic prophase in man. Cytogenet Cell Genet 38:241–247
Coen ES, Dover GA (1983) Unequal exchanges and the coevolution of X and Y rDNA arrays inDrosophila melanogaster. Cell 33:849–855
Committee for the Standardization of Chromosomes of Peromyscus (1977) Standardized karyotype of deer mice,Peromyscus (Rodentia). Cytogenet Cell Genet 19:38–43
Cooke HJ, Brown WR, Rappold GA (1985) Hypervariable telomeric sequences from the human sex chromosomes are pseudoautosomal. Nature 317:687–692
Counce SJ, Meyer GF (1973) Differentiation of the synaptonemal complex and the kinetochore inLocusta spermatocytes studied by whole mount electron microscopy. Chromosoma 44:231–253
Davis KM, Smith SA, Greenbaum IF (1986) Evolutionary implications of chromosomal polymorphisms in populations ofPeromyscus boylii from southwestern Mexico. Evolution 40:645–649
Evans EP, Breckon G, Ford CE (1964) An air-drying method of meiotic preparations from mammalian testes. Cytogenetics 3:289–294
Evans EP, Burtenshaw MD, Cattanach BM (1982) Meitoic crossing-over between the X and Y chromosomes of male mice carrying the sex-reversing (Sxr) factor. Nature 300:443–445
Goodfellow PJ, Darling SM, Thomas NS, Goodfellow PN (1986) A pseudoautosomal gene in man. Science 234:740–743
Greenbaum IF, Baker RJ (1978) Determination of the primative karyotype forPeromyscus. J Mammal 59:820–834
Greenbaum IF, Hale DW, Fuxa KP (1986) The mechanism of autosomal synapsis and the substaging of zygonema and pachynema from deer mouse spermatocytes. Chromosoma 93:203–212
Gunn SJ, Greenbaum IF (1986) Systematic implications of karyotypic variation in mainlandPeromyscus from the Pacific Northwest. J Mammal 67:294–304
Hale DW (1988) Cytogenetic analysis of chromosomal heterozygosity in thePeromyscus maniculatus species group. PhD Dissertation, Texas A&M University, College Station, p xi + 114
Hale DW, Greenbaum IF (1986) The behavior and morphology of the X and Y chromosomes during prophase I in the Sitka deer mouse (Peromyscus sitkensis). Chromosoma 94:235–242
Harbers K, Soriano P, Müller U, Jaenisch R (1986) High frequency of unequal recombination in pseudoautosomal region shown by proviral insertion in transgenic mouse. Nature 324:682–685
Hilwig I, Gropp A (1972) Staining of constitutive heterochromatin in mammalian chromosomes with a new fluorochrome. Expl Cell Res 75:122–126
Houseal TW, Greenbaum IF, Schmidly DJ, Smith SA, Davis KM (1987) Karyotypic variation inPeromyscus boylii from Mexico. J Mammal 68:281–296
Howell WM, Black DA (1980) Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experientia 36:1014
John B (1976) Myths and mechanisms of meiosis. Chromosoma 54:295–325
John B (1988) The biology of heterochromatin. In: Verma RS (ed) Heterochromatin: Molecular and structural aspects. Cambridge Univ Press, Cambridge, pp 1–147
Keitges EA, Schorderet DF, Gartler SM (1987) Linkage of the steroid sulfatase gene to the Sex-reversed mutation in the mouse. Genetics 116:465–468
Kurnit DM (1979) Satellite DNA and heterochromatin variants: The case for unequal mitotic crossing over. Hum Genet 47:169–186
Lee MR, Elder FFB (1980) Yeast stimulation of bone marrow mitosis for cytogenetic investigations. Cytogenet Cell Genet 26:36–40
Miklos GLG, John B (1979) Heterochromatin and satellite DNA in man: Properties and prospects. Am J Hum Genet 31:264–280
Moses MJ (1977) Synaptonemal complex karyotyping in spermatocytes of the chinese hamster (Cricetulus griseus) I. Morphology of the autosomal complement in spread preparations. Chromosoma 60:99–125
Moses MJ, Poorman PA, Roderick TH, Davisson MT (1982) Synaptonemal complex analysis of mouse chromosomal rearrangements IV. Synapsis and synaptic adjustment in two paracentric inversions. Chromosoma 84:457–474
Mürer-Orlando M, Richer CL (1983) Heterochromatin heterogeneity in Chinese hamster sex bivalents. Cytogenet Cell Genet 35:195–199
Page DC, de la Chapelle A, Weissenbach J (1986) Chromosome Y-specific DNA in related human XX males. Nature 315:224–226
Pathak S, Hsu TC, Arrighi FE (1973) Chromosomes ofPeromyscus (Rodentia, Cricetidae) IV. The role of heterochromatin in karyotype evolution. Cytogenet Cell Genet 12:315–326
Petit C, de la Chapelle A, Castillo S, Noël B, Weissenbach J (1987) An abnormal terminal X-Y interchange accounts for most but not all cases of human XX maleness. Cell 49:595–602
Rao SRV, Vasantha K, Thelma BK, Joyal RC, Jhanwar SC (1983) Heterochromatin variation and sex chromosome polymorphism inNesokia indica: A population study. Cytogenet Cell Genet 35:233–237
Rogers DS, Greenbaum IF, Gunn SJ, Engstrom MD (1984) Cytosystematic value of chromosomal inversion data in the genusPeromyscus (Rodentia: Cricetidae). J Mammal 65:457–465
Rouyer F, Simmler M-C, Johnsson C, Vergnaud G, Cooke HJ, Weissenbach J (1986) A gradient of sex linkage in the pseudoautosomal region of the human sex chromosomes. Nature 319:291–295
Satya-Prakash KL, Aswathanarayana NV (1984) Behavior of sex chromosomes during meiosis in the house shrew. J Hered 75:149–150
Schmid M, Johannisson R, Haaf T, Neitzel H (1987) The chromosomes ofMicromys minutus (Rodentia, Murinae). II. Pairing pattern of X and Y chromosomes in meiotic prophase. Cytogenet Cell Genet 45:121–131
Schmidly DJ, Cato P, Bradley RD (1988) Morphometric differentiation and taxonomy of chromosomally characterized populations ofPeromyscus boylii from east-central Mexico. J Mammal 69:462–480
Schweizer D, Ambros P, Andrle M (1978) Modification of DAPI banding on human chromosomes by prestaining with a DNA-binding oligopeptide antibiotic, distamycin A. Expl Cell Res 111:327–332
Seabright M (1971) A rapid banding technique for human chromosomes. Lancet ii:971–972
Sharma T, Raman R (1973) Variation of constitutive heterochromatin in the sex chromosomes of the rodentBandicota bengalensis bengalensis (Gray). Chromosoma 41:75–84
Smith GP (1976) Evolution of repeated DNA sequences by unequal crossing over. Science 191:528–535
Smith SA, Bradley RD, Greenbaum IF (1986) Karyotypic conservatism in thePeromyscus mexicanus group. J Mammal 67:584–586
Solari AJ (1974) The relationship between chromosomes and axes in the chiasmatic XY pair of the Armenian hamster (Cricetulus migratorius). Chromosoma 48:89–109
Soriano P, Keitges EA, Schorderet DF, Harbers K, Gartler SM, Jaenisch R (1987) High rate of recombination and double crossovers in the mouse pseudoautosomal region during male meiosis. Proc Natl Acad Sci USA 84:7218–7220
Stack S, Anderson L (1986) Two-dimensional spreads of synaptonemal complexes from solanaceous plants. III. Recombination nodules and crossing over inLycopersicon esculentum (tomato). Chromosoma 94:253–258
Stangl FB Jr, Baker RJ (1984) Evolutionary relationships in Peromyscus: congruence in chromosomal, genic and classical data sets. J Mammal 65:643–654
Sudman PD (1990) Cryogenic preservation of testicular material for synaptonemal complex analysis. Cytogenet Cell Genet 52:88–89
Sudman PD, Greenbaum IF (1989) Visualization of kinetochores in mammalian meiotic preparations and observations of argentophilic differences between mitotic and meiotic kinetochores. Genome 32:380–382
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304–306
Therman E, Kuhm EM (1976) Mitotic crossing-over and segregation in man. Hum Genet 59:93–100
Vistorin G, Gamperl R, Rosenkranz W (1977) Studies on sex chromosomes of four hamster species:Cricetus cricetus, Cricetulus griseus, Mesocricetus auratus, andPhodopus sungorus. Cytogenet Cell Genet 18:24–32
Wahlström J, Kyllerman M, Hansson A, Taranger J (1985) Unequal mitotic sister chromatid exchange and different length of Y chromosomes. Hum Genet 70:185–188
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sudman, P.D., Greenbaum, I.F. Unequal crossing over and heterochromatin exchange in the X-Y bivalents of the deer mouse,Peromyscus beatae . Chromosoma 99, 183–189 (1990). https://doi.org/10.1007/BF01731128
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01731128