Abstract
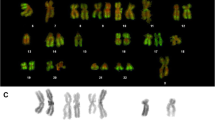
The distribution of Z-form DNA along the length of metaphase chromosomes of Indian muntjac was studied by indirect immunofluorescence procedures using an antibody specific to the Z-DNA conformation. Several fixation conditions were compared for reproducible detection of Z-DNA in isolated metaphase chromosomes. Fixation of chromosomes with 45% acetic acid alone gave reproducible reactivity with the antibody. When fixation was done either with Carnoy's solution (3:1 methanol:acetic acid) or with 75% alcohol alone, the antibody binding was at background level. Acetic acid-fixed chromosomes exhibited intense fluorescence both at C-band heterochromatin and at nucleolus organizer regions (NORs). The euchromatic regions had weakly, but clearly, stained bands, which were quite similar to the chromomycin A3 R-bands. After treatment with topoisomerase I, the immunofluorescence at NORs and R-bands disappeared, but only a slight decrease in immunofluorescence intensity was observed at C-band regions. We suggest that this difference in the immunoreactivity of NORs and R-bands from C-bands reflects a difference in gene activity among these regions. Possible molecular mechanisms involved in Z-DNA immunoreactivity are discussed, based on SDS-polyacrylamide gel electrophoretic analysis of chromosomal proteins after extraction of metaphase chromosomes with different fixative solutions.
Similar content being viewed by others
Abbreviations
- PI :
-
propidium iodide
- NOR(s) :
-
nucleolus organizer region(s)
- SDS-PAGE :
-
sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Arndt-Jovin DJ, Robert-Nicoud M, Zarling DA, Greider C, Weimer E, Jovin TM (1983) Left-handed Z-DNA in bands of acid-fixed polytene chromosomes. Proc Natl Acad Sci USA 80:4344–4348
Brat SV, Verma RS, Dosik H (1979) Structural organization of chromosomes of the indian muntjac (Muntiacus muntjak). Cytogenet Cell Genet 24:201–208
Burkholder GD, Latimer LJP, Lee JS (1988) Immunofluorescent staining of mammalian nuclei and chromosomes with a monoclonal antibody to triplex DNA. Chromosoma 97:185–192
Comings DE (1978) Mechanisms of chromosome banding and implications for chromosome structure. Annu Rev Genet 12:25–46
Hamada H, Kakunaga T (1982) Potential Z-DNA forming sequences are highly dispersed in the human genome. Nature 298:396–398
Haniford DB, Pulleyblank DE (1983) The in vivo occurence of Z-DNA. J Biomol Struct Dynam 1:593–609
Hill RJ, Stollar BD (1983) Dependence of Z-DNA antibody binding to polytene chromosomes on acid fixation and DNA torsional strain. Nature 305:338–340
Irie S, Sezaki M (1983) A quantitative determination of the relative amount of histones in polyacrylamide gel by silver stain. Anal Biochem 134:471–478
Jimenez-Ruiz A, Requena JM, Lancillotti F, Morales G, Lopez MC, Alonso C (1989) Molecular cloning of a Drosophila potential Z-DNA forming sequence hybridizing in situ to a developmentally regulated subdivision of the polytene chromosomes. Nucleic Acids Res 17:4579–4588
Kmiec EB, Angelides KJ, Holloman WK (1985) Left-handed DNA and the synaptic pairing reaction promoted by Ustilago rec1 protein. Cell 40:139–145
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lafer E, Sausa R, Rich A (1985) Anti-Z-DNA antibody binding can stabilize Z-DNA in relaxed and linear plasmids under physiological conditions. EMBO J 4:3655–3660
Lancillotti F, Lopez MC, Alonso C, Stollar BD (1985) Locations of Z-DNA in polytene chromosomes. J Cell Biol 100:1759–1766
Lancillotti F, Lopez MC, Arias P, Alonso C (1987) Z-DNA in transcriptionally active chromosomes. Proc Natl Acad Sci USA 84:1560–1564
Lemeunier F, Derbin C, Malfoy B, Leng M, Taillandier E (1982) Identification of left-handed Z-DNA by indirect immunofluorescence in polytene chromosomes ofChironomus thummi thummi. Exp Cell Res 141:508–513
Lipps HJ, Nordheim A, Lafer EM, Ammermann D, Stollar BD, Rich A (1983) Antibodies against Z DNA react with the macronucleus but not the micronucleus of the hypotrichous ciliateStylonychia mytilus. Cell 32:435–441
Morgenegg G, Celio MR, Malfoy B, Leng M, Kuenzle CC (1983) Z-DNA immunoreactivity in rat tissues. Nature 303:540–543
Nordheim A, Rich A (1983) Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature 303:674–679
Nordheim A, Pardue ML, Lafer EM, Moller A, Stollar BD, Rich A (1981) Antibodies to left-handed Z-DNA bind to interband regions ofDrosophila polytene chromosomes. Nature 294:417–422
Nordheim A, Tesser P, Azorin F, Kwon YH, Moller A, Rich A (1982) Isolation ofDrosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci USA 79:7729–7733
Pardue ML, Hsu TC (1975) Locations of 18S and 28S ribosomal genes on the chromosomes of the Indian muntjac. J Cell Biol 64:251–254
Pardue ML, Lowenhaupt K, Rich A, Nordheim A (1987) (dC-dA)n·(dG-dT)n sequences have evolutionarily conserved chromosomal locations in Drosophila with implications for roles in chromosome structure and function. EMBO J 6:1781–1789
Pulleyblank DE, Haniford DB, Morgan AR (1985) A structural basis for S1 nuclease sensitivity of double-stranded DNA. Cell 42:271–280
Rich A, Nordheim A, Wang AHJ (1984) The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem 53:791–846
Robert-Nicoud M, Arndt-Jovin DJ, Zarling DA, Jovin TM (1984) Immunological detection of left-handed Z DNA in isolated polytene chromosomes. Effects of ionic strength, pH, temperature and topological stress. EMBO J 3:721–731
Ueda T, Irie S, Kato Y (1987) Longitudinal differentiation of metaphase chromosomes of Indian muntjac as studied by restriction enzyme digestion, in situ hybridization with cloned DNA probes and distamycin A plus DAPI fluorescence staining. Chromosoma 95:251–257
Viegas-Pequignot E, Derbin C, Malfoy B, Taillandier E, Leng M, Dutrillaux B (1983) Z-DNA immunoreactivity in fixed metaphase chromosomes of primates. Proc Natl Acad Sci USA 80:5890–5894
Wang AH-J, Quigley GJ, Kolpak FJ, Crawford JL, van Boom JH, van der Marel G, Rich A (1979) Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282:680–686
Weinreb A, Collier D, Birshtein BK, Wells RD (1988) Intramolecular triplex and left-handed Z-DNA at a site of unequal sister chromatid exchange. J Cell Biol 107:p90a
Wittig B, Dorbic T, Rich A (1989) The level of Z-DNA in metabolically active, permealized mammalian cell nuclei is regulated by torsional strain. J Cell Biol 108:755–764
Wray W, Stubblefield E (1970) A new method for the rapid isolation of chromosomes, mitotic apparatus, or nuclei from mammalian fibroblasts at neutral pH. Exp Cell Res 59:469–478
Zarling DA, Arndt-Jobin DJ, Robert-Nicoud M, McIntosh LP, Thomae R, Jovin TM (1984) Immunoglobulin recognition of synthetic and natural left-handed Z-DNA conformations and sequences. J Mol Biol 176:369–377
Zimmerman SB (1982) The three-dimensional structure of DNA. Annu Rev Biochem 51:395–427
Author information
Authors and Affiliations
Additional information
Deceased, April 23, 1988
Rights and permissions
About this article
Cite this article
Ueda, T., Kato, Y. & Irie, S. Regional differences in immunostainability of isolated metaphase chromosomes of Indian muntjac with anti-Z-DNA antibody. Chromosoma 99, 161–168 (1990). https://doi.org/10.1007/BF01731126
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01731126