Summary
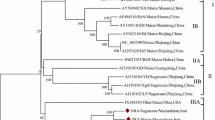
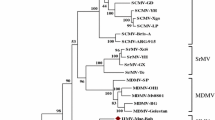
We have sequenced the NIb coding region of sugarcane mosaic potyvirus strain SC (SCMV-SC) and eight field isolates of SCMV from Australia. This region comprised 1563 nucleotides and encoded a putative protein of 521 amino acids containing the consensus motif GDD. The protease cleavage sites between the NIa/NIb and the NIb/coat protein were found to be Q/C and Q/A, respectively. The SCMV sequences were most similar to sorghum mosaic potyvirus with identities of 70% and 78% at the nucleotide and amino acid levels, respectively. When the sequences were compared to each other, there was a maximum of 3.3% variation between isolates at the nucleotide level and a maximum of 0.8% at the amino acid level. Phylogenetic analysis of the sequences indicated the field isolates were grouped according to their geographical location. The SCMV sequence with most homology to all other isolates has been selected to generate constructs for replicase-mediated resistance.
Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410
Audy P, Palukaitis P, Slack SA, Zaitlin M (1994) Replicase-mediated resistance to potato virus Y in transgenic tobacco plants. Mol Plant Microbe Interact 7: 15–22
Bateson MF, Henderson J, Chaleeprom W, Gibbs AJ, Dale JL (1994) Papaya ringspot virus: isolate variability and the origin of PRSV type P (Australia). J Gen Virol 75: 3547–3553
Brederode FT, Taschner PEM, Posthumus E, Bol JF (1995) Replicase mediated resistance to alfalfa mosaic virus. Virology 207: 467–474
Carr JP, Zaitlin M (1993) Replicase-mediated resistance. Semin Virol 4: 339–347
Donson J, Keamey CM, Turpen TH, Khan IA, Kurath G, Turpen AM, Jones GE, Dawson WO, Lewandowski DJ (1993) Broad resistance to tobamoviruses is mediated by a modified tobacco mosiac virus replicase transgene. Mol Plant Microbe Interact 6: 635–642
Farinelli L, Malnoe P, Collect GF (1992) Heterologous encapsidation of potato virus Y strain O (PVYO) with the transgenic coat protein of PVY strain N (PVYN) inSolanum tuberosum cv. Bintije. Bio/Technology 10: 1020–1025
Frenkel MJ, Jilka JM, McKern NM, Strike PM, Clark JM Jr, Shukla DD, Ward CW (1991) Unexpected sequence diversity in the amino-terminal ends of the coat proteins of strains of sugarcane mosaic virus. J Gen Virol 72: 237–242
Golemboski DB, Lomonosoff GF, Zaitlin M (1990) Plants transformed with a tobacco mosaic virus non-structural gene sequence are resistant to the virus. Proc Natl Acad Sci USA 87: 6311–6315
Koike H, Gillaspie AG (1989) Mosaic. In: Ricaud C, Egan BT, Gillaspie AG (eds) Diseases of sugarcane — major diseases. Elsevier, Amsterdam, pp 301–322
Lindbo JA, Dougherty WG (1992) Pathogen-derived resistance to a potyvirus: immune and resistant phenotypes in transgenic tobacco expressing altered forms of a potyvirus coat protein nucleotide sequence. Mol Plant Microbe Interact 5: 144–153
Longstaff M, Brigneti G, Boccard F, Chapman S, Baulcombe D (1993) Extreme resistance to potato virus X in plants expressing a modified component of the putative viral replicase. EMBO J 12: 379–386
Macfarlane SA, Davies JW (1992) Plants transformed with a region of the 201 kilodalton replicase gene from the pea-early browning virus RNA 1 are resistant to virus infection. Proc Natl Acad Sci USA 89: 5829–5833
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Shukla DD, Frenkel MJ, McKern NM, Ward CW, Jilka J, Tosic M, Ford RE (1992) Present status of the sugarcane mosaic subgroup of potyviruses. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 363–373 (Arch Virol [Suppl] 5)
Shukla DD, Tosic M, Jilka J, Ford RE, Toler RW, Langham MAC (1989) Taxonomy of potyviruses infecting maize, sorghum and sugarcane in Australia and the United States as determined by reactivities of polyclonal antibodies directed towards virus-specific N-termini of coat proteins. Phytopathology 79: 223–229
Shukla DD, Ward CW, Brunt AA (1994) The potyviridae. Centre for Agriculture and Biosciences International. Cambridge University Press, Cambridge
Smith GR, Van De Velde R (1994) Detection of sugarcane mosaic virus and Fiji disease virus in diseased sugarcane using the polymerase chain reaction. Plant Dis 78: 557–561
Smith GR, Ford R, Frenkel MJ, Shukla DD, Dale JL (1992) Transient expression of the coat protein of sugarcane mosaic virus in sugarcane protoplasts and expression inEscherichia coli. Arch Virol 125: 15–23
Stark DM, Beachy RN (1989) Protection against potyvirus infection in transgenic plants: evidence for broad spectrum resistance. Bio/Technology 7: 1257–1262
Teakle DS, Grylls NE (1973) Four strains of sugarcane mosaic virus infecting cereals and other grasses in Australia. Aust J Agric Res 24: 465–477
Tennant PF, Gonsalves C, Ling K-S, Fitch M, Manshardt R, Slightom JL, Gonsalves D (1994) Differential protection against papaya ringspot virus isolates in coat protein gene transgenic papaya and classically cross-protected papaya. Phytopathology 84: 1359–1366
Thompson JD, Higgins DJ, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680
Xiao XW, Frenkel MJ, Teakle DS, Ward CW, Shukla DD (1993) Sequence diversity in the surface exposed amino terminal region of the coat proteins of seven strains of SCMV correlates with their host range and other biological properties. Arch Virol 132: 399–408
Zaitlin M, Anderson JM, Perry KL, Zhang L, Palukaitis P (1994) Specificity of replicase-mediated resistance to cucumber mosaic virus. Virology 201: 100–205
Author information
Authors and Affiliations
Additional information
Sequence data deposited with Genbank, accession number U51455.
Rights and permissions
About this article
Cite this article
Handley, J.A., Smith, G.R., Dale, J.L. et al. Sequence diversity in the NIb coding region of eight sugarcane mosaic potyvirus isolates infecting sugarcane in Australia. Archives of Virology 141, 2289–2300 (1996). https://doi.org/10.1007/BF01718631
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01718631