Summary
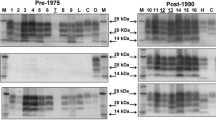
The structure of the scrapie agent remains unknown. However, scrapie infectivity tends to co-sediment with an infection specific fraction of the glycoprotein PrP (PrPSc) under conditions which solubilise the normal form of this protein (PrPc); accordingly, PrP has been proposed as a candidate component of the agent. To investigate this further we have been examining a new scrapie-related murine model in conjunction with established scrapie models. A bovine spongiform encephalopathy (BSE) derived murine model has short incubation periods, high infectivity titre and low amounts of PrP deposited in the brain. A membrane fraction from scrapie/BSE infected brain is solubilised with Sarkosyl at pH⩾9.0. Most PrP is also solubilised. In models of the disease with little deposition of PrP in the brain, this solubilisation step is particularly effective in reducing the amounts of PrP sedimented from brain extracts. Gradient centrifugation of the sedimented fraction shows further separation of infectivity and the residual PrP. It is concluded that at least some PrPSc in the brain need not be associated directly with infectious agent but is deposited in brain solely as a pathological product of infection. However, a residual sedimentable fraction contains PrP which may be a component of the agent.
Similar content being viewed by others
References
Bessen RA, Kocisko DA, Raymond GJ, Nandan S, Lansbury PT, Caughey B (1995) Non-genetic propagation of strain-specific properties of scrapie prion protein. Nature 375: 698–700
Brown P, Liberski PP, Wolff A, Gajdusek DC (1990) Conservation of infectivity in purified fibrillary extracts of scrapie-infected hamster brain after sequential enzymatic digestion or polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 87: 7240–7244
Bruce ME, Dickinson AG (1987) Biological evidence that scrapie agent has an independent genome. J Gen Virol 68: 79–89
Bruce ME, Fraser H (1975) Amyloid plaques in the brains of mice infected with scrapie: morphological variation and staining properties. Neuropathol Appl Neurobiol 1: 189–202
Bruce ME, McBride PA, Farquhar CF (1989) Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett 102: 1–6
Bruce ME, McConnell I, Fraser H, Dickinson AG (1991) The disease characteristics of different strains of scrapie inSinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol 72: 595–603
Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347
Carlson GA, Kingsbury DT, Goodman PA, Coleman S, Marshall ST, DeArmond S, Westaway D, Prusiner SB (1986) Linkage of prion protein and scrapie incubation time genes. Cell 46: 503–511
Caughey B, Raymond GJ (1991) The scrapie-associated form of PrP is made from a cell-surface precursor that is both protease-sensitive and phospholipase-sensitive. J Biol Chem 266: 18217–18223
Chasteen ND, Theil EC (1982) Iron binding by horse spleen apoferritin. J Biol Chem 257: 7672–7677
Clarke MC, Millson GC (1976) The membrane location of scrapie infectivity. J Gen Virol 31: 441–445
Czub M, Braig HR, Diringer H (1986) Pathogenesis of scrapie: study of the temporal development of clinical symptoms, of infectivity titres and scrapie-associated fibrils in brains of hamsters infected intraperitoneally. J Gen Virol 67: 2005–2009
Czub M, Braig HR, Diringer H (1988) Replication of the scrapie agent in hamsters infected intracerebrally confirms the pathogenesis of an amyloid-inducing virosis. J Gen Virol 69: 1753–1756
DeArmond SJ, McKinley MP, Barry RA, Braunfeld MB, McColloch JR, Prusiner SB (1985) Identification of prion amyloid filaments in scrapie brain. Cell 41: 221–235
DeArmond SJ, Mobley WC, DeMott DL, Barry RA, Beckstead JH, Prusiner SB (1987) Changes in the localization of brain prion proteins during scrapie infection. Neurology 37: 1271–1280
Dickson AG (1976) Scrapie in sheep and goats. Front Biol 44: 209–241
Dickinson AG, Meikle VM (1971) Host-genotype and agent effects in scrapie incubation: change in allelic interaction with different strains of agent. Mol Gen Genet 112: 73–79
Dickinson AG, Meikle VM, Fraser H (1968) Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J Comp Pathol 78: 293–299
Dickinson AG, Outram GW (1979) The scrapie replication-site hypothesis and its implications for pathogenesis. In: Prusiner SB, Hadlow WJ (eds) Slow transmissible diseases of the central nervous system, vol 2. Academic Press, New York, pp 13–32
Diringer H, Gelderblom H, Hilmert H, Ozel M, Edelbluth C, Kimberlin RH (1983) Scrapie infectivity, fibrils and low molecular weight protein. Nature 306: 476–478
Dougherty RM (1964) Animal virus titration techniques. In: Harris RJC (eds) Techniques in experimental virology. Academic Press, London, pp 169–223
Farquhar CF, Somerville RA, Ritchie LA (1989) Post-mortem immunodiagnosis of scrapie and bovine spongiform encephalopathy. J Virol Methods 24: 215–221
Fraser H, McConnell I, Wells GAH, Dawson M (1988) Transmission of bovine spongiform encephalopathy to mice. Vet Rec 123: 472
Haig DA, Clarke MC (1965) Observations on the agent of scrapie. In: Gajdusek DC, Gibbs CJ, Alpers M (eds) Slow, latent and temperate virus infections, vol 2. U.S. Government Printing Office, Washington, pp 215–219
Hope J, Morton LJD, Farquhar CF, Multhaup G, Beyreuther K, Kimberlin RH (1986) The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge-distribution andN-terminal protein-sequence as predicted for the normal brain protein (PrP). Embo J 5: 2591–2597
Hope J, Multhaup G, Reekie LJ, Kimberlin RH, Beyreuther K (1988) Molecular pathology of scrapie-associated fibril protein (PrP) in mouse brain affected by the ME7 strain of scrapie. Eur J Biochem 172: 271–277
Hunter GD, Kimberlin RH, Millson GC, Gibbons RA (1971) An experimental examination of the scrapie agent in cell membrane mixtures. I. Stability and physicochemical properties of the scrapie agent. J Comp Pathol 81: 23–32
Hunter N, Hope J, McConnell I, Dickinson AG (1987) Linkage of the scrapie-associated fibril protein (PrP) gene andSinc using congenic mice and restriction fragment length polymorphism analysis. J Gen Virol 68: 2711–2716
Jeffrey M, Goodsir CM, Bruce M, McBride PA, Scott JR, Halliday WG (1994) Correlative light and electron-microscopy studies of PrP localization in 87V scrapie. Brain Res 656: 329–343
Kimberlin RH, Walker C (1977) Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol 34: 295–304
Kimberlin RH, Walker CA, Millson GC, Taylor DM, Robertson PA, Tomlinson AH, Dickinson AG (1983) Disinfection studies with 2 strains of mouse-passaged scrapie agent — guidelines for Creutzfeldt-Jakob and related agents. J Neurol Sci 59: 355–369
Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, Lansbury PT, Caughey B (1994) Cell-free formation of protease-resistant prion protein. Nature 370: 471–474
Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J (1994) 129/ola mice carrying a null mutation in PrP that abolishes messenger-RNA production are developmentally normal. Mol Neurobiol 8: 121–127
Manson J, McBride P, Hope J (1992) Expression of the PrP gene in the brain ofSinc congenic mice and its relationship to the development of scrapie. Neurodegen 1: 45–52
Manuelidis L, Sklaviadis T, Manuelidis EE (1987) Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. Embo J 6: 341–347
McBride PA, Bruce ME, Fraser H (1988) Immunostaining of scrapie cerebral amyloid plaques with antisera raised to scrapie-associated fibrils (SAF). Neuropathol Appl Neurobiol 14: 325–336
McKenzie D, Kaczkowski J, Marsh R, Aiken J (1994) Amphotericin-B delays both scrapie agent replication and PrP-res accumulation early in infection. J Virol 68: 7534–7536
McKinley MP, Bolton DC, Prusiner SB (1983) A protease-resistant protein is a structural component of the scrapie prion. Cell 35: 57–62
Merz PA, Somerville RA, Wisniewski HM, Iqbal K (1981) Abnormal fibrils from scrapie-infected brain. Acta Neuropathol 54: 63–74
Meyer RK, McKinley MP, Bowman KA, Braunfeld MB, Barry RA, Prusiner SB (1986) Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci USA 83: 2310–2314
Millson GC, Hunter GD, Kimberlin RH (1971) An experimental examination of the scrapie agent in cell membrane mixtures. II. The association of scrapie activity with membrane fractions. J Comp Pathol 81: 255–265
Neville DM (1971) Molecular weight determination of protein-dodecyl sulphate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem 246: 6328–6334
Prusiner SB, Groth DF, McKinley MP, Cochran SP, Bowman KA, Kasper KC (1981) Thiocyanate and hydroxylions inactivate the scrapie agent. Proc Natl Acad Sci USA 78: 4606–4610
Prusiner SB, Scott M, Foster D, Pan K-M, Groth D, Mirenda C, Torchia M, Yang S-L, Serban D, Carlson GA, Hoppe PC, Westaway D, DeArmond SJ (1990) Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63: 673–686
Rubenstein R, Carp RI, Ju W, Scalici C, Papini M, Rubenstein A, Kascsak R (1994) Concentration and distribution of infectivity and PrPSc following partial denaturation of a mouse-adapted and a hamster-adapted scrapie strain. Arch Virol 139: 301–311
Rubenstein R, Merz PA, Kascsak RJ, Scalici CL, Papini MC, Carp RI, Kimberlin RH (1991) Scrapie-infected spleens-analysis of infectivity, scrapie-associated fibrils, and protease-resistant proteins. J Infect Dis 164: 29–35
Safar J, Wang W, Padgett MP, Ceroni M, Piccardo P, Zopf D, Gajdusek DC, Gibbs CJ (1990) Molecular mass, biochemical composition, and physicochemical behavior of the infectious form of the scrapie precursor protein monomer. Proc Natl Acad Sci USA 87: 6373–6377
Sakaguchi S, Katamine S, Yamanouchi K, Kishikawa M, Moriuchi R, Yasukawa N, Doi T, Miyamoto T (1993) Kinetics of infectivity are dissociated from PrP accumulation in salivary glands of Creutzfeldt-Jakob disease agent-inoculated mice. J Gen Virol 74: 2117–2123
Sammons DW, Adams LD, Nishizawa EE (1981) Ultrasensitive silver-based colour staining of polypeptides in polyacrylamide gels. Electrophoresis 2: 135–141
Scott JR, Davies D, Fraser H (1992) Scrapie in the central nervous system: neuroanatomical spread of infection andSinc control of pathogenesis. J Gen Virol 73: 1637–1644
Scott JR, Reekie LJD, Hope J (1991) Evidence for intrinsic control of scrapie pathogenesis in the murine visual-system. Neurosci Lett 133: 141–144
Scott M, Foster D, Mirenda C, Serban D, Coufal F, Walchli M, Torchia M, Groth D, Carlson G, DeArmond SJ, Westaway D, Prusiner SB (1989) Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59: 847–857
Sklaviadis T, Akowitz A, Manuelidis EE, Manuelidis L (1990) Nuclease treatment results in high specific purification of Creutzfeldt-Jakob disease infectivity with a density characteristic of nucleic acid-protein complexes. Arch Virol 112: 215–228
Sklaviadis TK, Manuelidis L, Manuelidis EE (1989) Physical properties of the Creutzfeldt-Jakob disease agent. J Virol 63: 1212–1222
Somerville RA (1991) The transmissible agent causing scrapie must contain more than protein. Rev Med Virol 1: 131–139
Somerville RA, Carp RI (1983) Altered scrapie infectivity estimates by titration and incubation period in the presence of detergents. J Gen Virol 64: 2045–2050
Somerville RA, Merz PA, Carp RI (1986) Partial copurification of scrapie-associated fibrils and scrapie infectivity. Intervirol 25: 48–55
Somerville RA, Ritchie LA, Gibson PH (1989) Structural and biochemical evidence that scrapie-associated fibrils assemble in vivo. J Gen Virol 70: 25–35
Stahl N, Borchelt DR, Hsiao K, Prusiner SB (1987) Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51: 229–240
Tateishi J, Tashima T, Kitamoto T (1991) Practical methods for chemical inactivation of Creutzfeldt-Jakob disease pathogen. Microbiol Immunol 35: 163–166
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB (1987) Distinct prion proteins in short and long scrapie incubation period mice. Cell 51: 651–662
Xi YG, Ingrosso L, Ladogana A, Masullo C, Pocchiari M (1992) Amphotericin B treatment dissociates in vivo replication of the scrapie agent from PrP accumulation. Nature 356: 598–601
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Somerville, R.A., Dunn, A.J. The association between PrP and infectivity in scrapie and BSE infected mouse brain. Archives of Virology 141, 275–289 (1996). https://doi.org/10.1007/BF01718399
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01718399