Summary
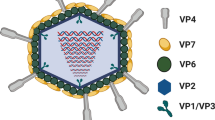
Bovine-human reassortant strains containing ten human rotavirus gene segments and segment 4, encoding VP4, of a bovine rotavirus were isolated from the stool of an infected Bangladeshi infant during cell culture adaptation. Two plaque purified variants of this reassortant, one making very large (429-L4) and the other tiny (429-S4) plaques, were further analyzed. The electropherotypes of these variants were identical except for slight mobility differences in segment 4. The predicted sequence of amino acids (aa) 16–280 in VP4 proteins revealed four differences between variants even in this limited region, so no single difference could be linked to plaque size. The small plaque variant S4 was phenotypically unstable and mutated to a large plaque-former within a single cell culture passage. The predicted sequence of aa 16–280 of a large plaque variant derived from S4 revealed six changes, only one of which was common to that of the L4 strain, thus suggesting that multiple amino acid changes in VP4 may affect plaque size. Although the large plaque variant L4 grew faster and was released from cells more rapidly than S4, its replication and that of other rotaviruses tested (i.e. RRV, NCDV and Wa) was suppressed by S4 in coinfected cells. Using an RRV×S4 reassortant containing only RRV segment 4, it was established that suppression was linked to the S4 VP4 protein. This suppression could not be associated with inhibition of viral adsorption and, therefore, appeared to occur following internalization. Thus, a new property of the rotavirus VP4 protein has been identified in a bovine-human rotavirus reas-sortant.
Similar content being viewed by others
References
Bass DM, Mackow ER, Greenberg HB (1991) Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology 183: 602–610
Burke B, Bridger JC, Desselberger U (1994) Temporal correlation between a single amino acid change on the VP4 of a porcine rotavirus and a marked change in pathogenicity. Virology 202: 754–759
Das M, Dunn SJ, Woode GN, Greenberg HB, Rao CD (1993) Both surface proteins (VP4 and VP7) of a asymptomatic neonatal rotavirus strain (I321) have high levels of sequence identity with the homologous proteins of a serotype 10 bovine rotavirus. Virology 194: 374–379
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387–395
Dunn SJ, Ward RL, McNeal MM, Cross TL, Greenberg HB (1993) Identification of a new neutralization epitope on VP7 of human serotype 2 rotavirus and evidence for electropherotype differences caused by single nucleotide substitutions. Virology 197: 397–404
Estes MK, Graham DY, Mason BB (1981) Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol 39: 879–888
Estes MK, Cohen J (1989) Rotavirus gene structure and function. Microbiol Rev 53: 410–449
Fukudome K, Yoshie O, Konno T (1989) Comparison of human, simian and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology 172: 196–205
Gentsch JR, Das BK, Jiang B, Bhan MK, Glass RI (1993) Similarity of the VP4 protein of human rotavirus strain 116E to that of the bovine B233 strain. Virology 194: 424–430
Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, Das BK, Bhan MK (1992) Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol 30: 1365–1373
Gorziglia M, Larralde G, Ward RL (1990) Neutralization epitopes on rotavirus SA11 4fM outer capsid proteins. J Virol 64: 4534–4539
Gorziglia M, Green K, Nishikawa K, Taniguchi K, Jones R, Kapikian AZ, Chanock RM (1988) Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J Virol 62: 2978–2984
Kalica AR, Flores J, Greenberg HB (1983) Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology 125: 194–205
Kitamoto N, Mattion NM, Estes MK (1993) Alterations in the sequence of the gene 4 from a human rotavirus after multiple passages in HepG2 liver cells. Arch Virol 130: 179–185
Lusso P, Secchiero P, Crowley RW, Garzino-Demo A, Berneman ZN, Gallo RC (1994) CD4 is a critical component of the receptor for human herpesvirus 7: interference with human immunodeficiency virus. Proc Natl Acad Sci USA 91: 3872–3876
Mackow ER, Barrett JW, Chan H, Greenberg HB (1989) The rhesus rotavirus outer capsid protein VP4 functions as a hemagglutinin and is antigenically conserved when expressed by a baculovirus recombinant. J Virol 63: 1661–1668
Nakagomi O, Nakogomi T (1993) Interspecies transmission of rotaviruses studied from the perspective of genogroup. Microbiol Immunol 37: 337–348
Nakagomi O, Isegawa Y, Ward RL, Knowlton DR, Kaga E, Nakagomi T, Ueda S (1994) Naturally occurring dual infection with human and bovine rotaviruses as suggested by the recovery of G1P8 and G1P5 rotaviruses from a single patient. Arch Virol 137: 381–388
Nishikawa K, Taniguchi K, Torres A, Hoshino Y, Green K, Kapikian AZ, Chanock RM, Gorziglia M (1988) Comparative analysis of the VP3 gene of divergent strains of the rotaviruses simian SA11 and bovine Nebraska calf diarrhea virus. J Virol 62: 4022–4026
Offit PA, Blavat G, Greenberg HB, Clark HF (1986) Molecular basis of rotavirus virulence: role of gene segment 4. J Virol 57: 46–49
Pongsuwanna Y, Taniguchi K, Choonthanom M, Chiwakul M, Jayavasu C, Snodgrass DR, Urasawa S (1990) Serological and genetic characterization of bovine rotaviruses in Thailand by ELISA and RNA-RNA hybridization: detection of numerous non-serotype 6 strains. Southeast Asian J Trop Med Publ Health 21: 607–613
Prasad BVV, Wang GJ, Clerx JPM, Chiu W (1988) Three-dimensional structure of rotavirus. J Mol Biol 199: 269–275
Prasad BVV, Chui W (1994) Structure of rotavirus. In: Ramig RF (ed) Rotaviruses. Curr Top Microbiol Immunol 185: 9–29
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Schiff LA, Fields BN (1990) Reoviruses and their replication. In: Fields BN, Knipe DM, Chanock RM, Hirsch MS, Melnick JL, Monath TP, Roizman B (eds) Field's Virology, 2nd ed. Raven Press, New York, pp 1275–1306
Shaw AL, Rothnagel R, Chen D, Ramig RF, Chiu W, Prasad BVV (1993) Three-dimensional visualization of the rotavirus hemagglutinin structure. Cell 74: 693–701
Taniguchi K, Nishikawa K, Kobayashi N, Urasawa T, Wu H, Gorziglia M, Urasawa S (1994) Differences in plaque size and VP4 sequence found in SA11 virus clones having simian authentic VP4. Virology 198: 325–330
Taniguchi K, Urasawa T, Urasawa S (1993) Independent segregation of the VP4 and the VP7 genes in bovine rotaviruses as confirmed by VP4 sequence analysis of G8 and G10 bovine rotavirus strains. J Gen Virol 74: 1215–1221
Urasawa S, Hasegawa A, Urasawa T, Taniguchi K, Wakasugi F, Suzuki H, Inoue S, Pongprot B, Supawadee J, Suprasent S, Rangsiyanond P, Tonusin S, Yamazi Y (1992) Antigenic and genetic analyses of human rotaviruses in Chiang Mai, Thailand: evidence for a close relationship between human and animal rotaviruses. J Infect Dis 166: 227–234
Ward RL, Knowlton DR, Pierce MJ (1984) Efficiency of human rotavirus propagation in cell culture. J Clin Microbiol 19: 748–753
Ward RL, Knowlton DR, Hurst P-FL (1988) Reassortant formation and selection following coinfection of cultured cells with subgroup 2 human rotaviruses. J Gen Virol 69: 149–162
Ward RL, Clemens JD, Sack DA, Knowlton DR, McNeal MM, Huda N, Ahmed F, Rao M, Schiff GM (1991) Culture adaption and characterization of group A rotaviruses causing diarrheal illnesses in Bangladesh from 1985 to 1986. J Clin Microbiol 29: 1915–1923
Ward RL, McNeal MM, Clemens JD, Sack DA, Rao M, Huda N, Green KY, Kapikian AZ, Coulson BS, Bishop RF, Greenberg HB, Gerna G, Schiff GM (1991) Reactivities of serotyping monoclonal antibodies with culture-adapted human rotaviruses. J Clin Microbiol 29: 449–456
Ward RL, Nakagomi O, Knowlton DR, McNeal MM, Nakagomi T, Clemens JD, Sack DA, Schiff GM (1990) Evidence for natural reassortants of human rotaviruses belonging to different genogroups. J Virol 64: 3219–3225
Yeager M, Dryden KA, Olson NH, Greenberg HB, Baker TS (1990) Three-dimensional structure of rhesus rotavirus by cryoelection microscopy and image reconstruction. J Cell Biol 110: 2133–2144
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ward, R.L., Jin, Q., Nakagomi, O. et al. Isolation of a human rotavirus containing a bovine rotavirus VP4 gene that suppresses replication of other rotaviruses in coinfected cells. Archives of Virology 141, 615–633 (1996). https://doi.org/10.1007/BF01718321
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01718321