Summary
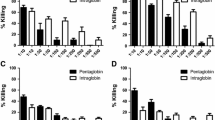
Intestinal infection byEscherichia coli O157 and other verotoxin (VT) producingE. coli has been increasingly recognized as an important factor for the causation of classic (enteropathic) hemolytic uremic syndrome (HUS) and hemorrhagic colitis (HC). Toxins most frequently involved are VT1 and VT2. As with other toxin-mediated diseases, administration of immunoglobulin (Ig) may be beneficial. However, little is known about the immune response elicited by the toxin(s), and the prevalence of VT neutralizing antibodies in the healthy population. We studied the capacity of seven Igs and a commercial plasma preparation to neutralize four different VTs (VT1, VT2, VT2c and VT2e). The results were compared with the neutralization titers (NT50%) of normal human serum samples from various age groups. Plasma products and normal sera were separated by protein G affinity chromatography to investigate the factor(s) responsible for VT neutralization. All Igs neutralized VT1 (8 to 96 NT50%). None of them inhibited VT2, VT2c or VT2e effectively. In contrast, none of 40 pediatric, and only one of 20 adult control sera (starting dilution 1 : 4) neutralized VT1 (25 NT50%). All 60 samples as well as the plasma preparation blocked VT2 (22 to 446 NT50%, median 137), but not VT2c and VT2e. The VT1 neutralizing activity was eluted with the IgG fraction. The VT2 neutralizing activity was not bound by protein G, but was recovered in the IgG-free effluent. In conclusion, therapeutic Igs significantly neutralize VT1, but are largely ineffective against other VTs. In contrast, all control sera inhibited VT2, but rarely VT1. Different principles, notably IgG and non-IgG (probably non-immunoglobulin) factors, respectively, appear to be responsible for the reduction of VT1 and VT2 cytotoxicityin vitro. Patients with VTEC disease are rarely expected to benefit from the use of presently available Igs.
Zusammenfassung
Enterale Infektionen durch Verotoxin-(VT-)produzierendeEscherichia coli werden zunehmend als Ursache des klassischen hämolytisch-urämischen Syndroms (HUS) erkannt. Das HUS ist häufig mit VT2 allein, seltener mit VT1 und VT2 assoziiert. Wie bei anderen bakteriell-toxischen Erkrankungen könnten Immunglobuline (Ig) von therapeutischem Nutzen sein. Die VT-induzierte Immunantwort und die Prävalenz neutralisierender Antikörper in der gesunden Bevölkerung sind jedoch nur unzureichend bekannt. Wir untersuchten die VT-Neutralisationstiter (NT50%) von sieben Ig- und einem kommerziellen Plasmapräparat gegenüber VT1, VT2, VT2c und VT2e und verglichen sie mit normalen Serumproben (NHS) aus verschiedenen Altersstufen. Zur näheren Charakterisierung der neutralisierenden Aktivität wurden das Plasmapräparat und NHS über eine Protein-G-Säule chromatographisch aufgetrennt. Alle Ig-neutralisierten VT1 (8–96 NT50%), jedoch nicht VT2, VT2c oder VT2e. Andererseits wurde VT1 von keinem der 40 pädiatrischen und nur von einem von 20 adulten Kontrollseren neutralisiert (25 NT50%; Anfangsverdünnung 1 : 4). Alle 60 Serumproben und die Plasmakonserve blockierten VT2 (22–446 NT50%, Median 137), aber nicht VT2c oder VT2e. Die VT1-neutralisierende Aktivität wurde mit der IgG-Fraktion eluiert, während die VT2-neutralisierende Aktivität im IgG-freien Durchlauf lokalisiert wurde. Therapeutische Ig neutralisieren VT1in vitro, sind aber weitgehend unwirksam gegen andere VT. Im Gegensatz dazu inhibieren Seren aller Altersgruppen VT2, aber selten VT1. Wahrscheinlich sind unterschiedliche Wirkprinzipien für die Neutralisation von Verotoxinen durch normale Seren verantwortlich, nämlich IgG für VT1 und eine Nicht-Immunglobulinfraktion für VT2. Bei der Mehrzahl der HUS-Patienten ist von der Gabe derzeit verfügbarer Ig-Präparate ein VT-spezifischer therapeutischer Effekt nicht zu erwarten.
Similar content being viewed by others
References
Karmali, M. A. Infection by verocytotoxin-producingEscherichia coli. Clin. Microbiol. Rev. 2 (1989) 15–38.
Richardson, S. E., Rotman, T. A., Jay, V., Smith, C. R., Becker, L. E., Petric, M., Olivieri, N. R., Karmali, M. A. Experimental verocytotoxemia in rabbits. Infect. Immun. 60 (1992) 4154–4167.
MacLeod, D. L., Gyles, C. L., Wilcox, B. P. Reproduction of edema disease of swine with purified Shiga-like toxin-II variant. Vet. Pathol. 28 (1991) 66–73.
Fong, J. S. C., de Chadarevian, J. P., Kaplan, B. Hemolytic uremic syndrome. Current concepts and management. Pediatr. Clin. North Am. 29 (1984) 835–856.
Richardson, S. E., Karmali, M. A., Becker, L. E., Smith, C. R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producingEscherichia coli infections. Hum. Pathol. 19 (1988) 1102–1108.
Argyle, J. C., Hogg, R. J., Pysher, T. J., Silva, F. G., Siegler, R. L. A clinicopathological study of 24 children with hemolytic uremic syndrome. Pediatr. Nephrol. 4 (1990) 52–58.
Griffin, P. W., Tauxe, R. V. The epidemiology of infections caused byEscherichia coli O157: H7, other enterohemorrhagicE. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13 (1991) 60–98.
O'Brien, A. D., Holmes, R. K. Shiga and Shiga-like toxins. Microbiol. Rev. 51 (1987) 206–220.
Ito, H., Terai, A., Kurazono, H., Takeda, Y., Nishibuchi, M. Cloning and nucleotide sequencing of Verotoxin 2 variant genes fromEscherichia coli O91: H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathogen. 8 (1990) 47–60.
Meyer, T., Karch, H., Hacker, J., Bocklage, H., Heesemann, J. Cloning and sequencing of a Shiga-like toxin II-related gene fromEscherichia coli O157: H7 strain 7279. Zentralbl. Bakt. Int. J. Microbiol. 276 (1992) 176–188.
Gannon, V. P. J., Teerling, C., Masri, S. A., Gyles, C. L. Molecular cloning and nucleotide sequence of another variant of theEscherichia coli Shiga-like toxin II family. J. Gen. Microbiol. 136 (1990) 1125–1135.
Linggood, M. A., Thompson, J. M. Verotoxin production among porcine strains ofEscherichia coli and its association with oedema disease. J. Med. Microbiol. 25 (1987) 359–362.
O'Brien, A. D., Tesh, V. L., Donohue-Rolfe, A., Jackson, M. P., Olsnes, S., Sandvig, K., Lindberg, A. A., Keusch, G. T. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180 (1992) 65–94.
Lingwood, C. A., Law, H., Richardson, S., Petric, M., Brunton, J. L., DeGrandis, S., Karmali, M. Glycolipid binding of purified and recombinantEscherichia coli produced verotoxinin vitro. J. Biol. Chem. 262 (1987) 8834–8839.
Obrig, T. G., Vecchio, P. J. D., Brown, J. E., Moran, T. P., Rowland, B. M., Judge, T. K., Rothman, S. W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect. Immun. 56 (1988) 2373–2378.
Barley-Maloney, L., Obrig, T., Daniel, T. Human renal microvascular endothelial cells (HRMEC) are targets for hemolytic uremic syndrome (HUS)-associated verotoxin (VT). J. Am. Soc. Nephrol. 1 (1990) 515.
van de Kar, N. C. A. J., Monnens, L. A. H., Karmali, M. A., van Hinsbergh, V. W. M. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood 80 (1992) 2755–2764.
Head, S. C., Karmali, M. A., Roscoe, M. E., Petric, M., Strockbine, N. A., Wachsmuth, I. K. Serological differences between verocytotoxin 2 and Shiga-like toxin II. Lancet. ii (1988) 751.
Weinstein, D. L., Jackson, M. P., Samuel, J. E., Holmes, R. K. Cloning and sequencing of a Shiga-like toxin type II variant from anEscherichia coli strain responsible for edema disease of swine. J. Bacteriol. 170 (1988) 4223–4230.
Hii, J. H., Gyles, C., Morooka, T., Karmali, M. A., Clarke, R., deGrandis, S., Brunton, J. L. Development of Verotoxin 2- and Verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v fromEscherichia coli E32511 and B2F1. J. Clin. Microbiol. 29 (1991) 2704–2709.
Schmitt, C. K., McKee, M. L., O'Brien, A. D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagicEscherichia coli strains are responsible for the antigenic heterogeneity of the O157: H-strain E32511. Infect. Immun. 59 (1991) 1065–1073.
Downes, F. P., Barrett, T. J., Green, J. H., Aloisio, C. H., Spika, J. S., Strockbine, N. A., Wachsmuth, I. K. Affinity purification and characterization of Shiga-like toxin II and production of toxin-specific monoclonal antibodies. Infect. Immun. 56 (1988) 1926–1933.
Boyd, B., Richardson, S., Gariépy, J. Serological responses to the B subunit of shiga-like toxin 1 and its peptide fragments indicate that the B subunit is a vaccine candidate to counter the action of the toxin. Infect. Immun. 59 (1991) 750–757.
Bitzan, M., Ludwig, K., Klemt, M., Koenig, H., Büren, J., Müller-Wiefel, D. E. The role ofEscherichia coli O157 infections in the classical (enteropathic) haemolytic uraemic syndrome: results of a Central European, multicentre study. Epidemiol. Infect. 110 (1993) 183–196.
Karch, H., Bitzan, M., Pietsch, R., Stenger, K., Wulffen, H. V., Heesemann, J., Düsing, R. Purified verotoxins ofEscherichia coli O157: H7 decrease prostacyclin synthesis by endothelial cells. Microb. Pathogen. 5 (1988) 215–221.
Meyer, T., Bitzan, M., Sandkamp, O., Karch, H. Synthetic oligode-oxyribonucleotide probes to detect verocytotoxin-producing-Escherichia coli in diseased pigs. FEMS Microbiol. Lett. 57 (1989) 247–252.
Head, S. C., Petric, M., Richardson, S., Roscoe, M., Karmali, M. A. Purification and characterization of verocytotoxin 2. FEMS Microbiol. Lett. 51 (1988) 211–216.
Tyrrell, G. J., Ramotar, K., Toye, B., Boyd, B., Lingwood, C. A., Brunton, J. L. Alteration of the carbohydrate binding specificity of verotoxins from Gal alpha1–4Gal to GalNacβ1–3 Gal alpha1–4 Gal and vice-versa by site-directed mutagenesis of the binding subunit. Proc. Natl. Acad. Sci. USA. 89 (1992) 524–528.
Marques, L. R. M., Peiris, J. S. M., Cryz, J. S., O'Brien, A. D. Escherichia coli strains isolated from pigs with edemea disease produce a variant of Shiga-like toxin II. FEMS Microbiol. Lett. 44 (1987) 33–38.
DeGrandis, S., Law, H., Brunton, J., Gyles, C., Lingwood, C. A. Globotetraosylceramide is recognized by the pig edema disease toxin. J. Biol. Chem. 264 (1989) 12520–12525.
Samuel, J. E., Perera, L. P., Ward, S., O'Brien, A. D., Ginsburg, V., Krivan, H. C. Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect. Immun. 58 (1990) 611–618.
Gentry, M. K., Dalrymple, J. M. Quantitative microtiter assay for Shigella toxin. J. Clin. Microbiol. 12 (1980) 361–366.
Klemt, M., Bitzan, M. Prevalence of shiga-like toxin neutralizing antibodies in children with hemolytic-uremic syndrome (HUS) and healthy controls. Zentralbl. Bakt. Int. J. Microbiol. 273 (1990) 101.
Weber, E. Grundriß der biologischen Statistik. Gustav Fischer Verlag, Jena 1980, pp. 232–240, 337–340.
Tarr, P. I., Neill, M. A., Clausen, C. R., Newland, J. W., Neill, R. J., Moseley S. L. Genotypic variation in pathogenicEscherichia coli O157: H57 isolated from patients in Washington, 1984–1987. J. Infect. Dis. 159 (1989) 344–347.
Kleanthous, H., Smith, H. R., Scotland, S. M., Gross, R. J., Rowe, B., Taylor, C. M., Milford, D. V. Haemolytic uraemic syndrome in the British Isles, 1985–1988: association with verocytotoxin producingEscherichia coli. Part 2: microbiological aspects. Arch. Dis. Child. 65 (1990) 722–727.
Willshaw, G. A., Scotland, S. M., Smith, H. R., Rowe, B. Properties of vero cytotoxin-producingEscherichia coli of human origin of O serogroups other than O157. J. Infect. Dis. 166 (1992) 797–802.
Bockemühl, J., Aleksic, S., Karch, H. Serological and biochemical properties of Shiga-like toxin (verotoxin)-producing strains ofEscherichia coli, other than O-group 157, from patients in Germany. Zentralbl. Bakt. Int. J. Microbiol. 276 (1992) 189–195.
Bitzan, M., Karch, H., Maas, M., Meyer, T., Rüssmann, H., Aleksic, S., Bockemühl, J. Clinical and genetic aspects of Shiga-like toxin production and traditional enteropathogenicEscherichia coli. Zentralbl. Bakt. Int. J. Med. Microbiol. 274 (1991) 496–506.
Bitzan, M., Karch, H., Klemt, M., Büren, J., Altrogge, H. Relevance of verotoxin mediated hemolytic-uremic syndrome in Germany. Nieren Hochdruck. 17 (1988) 246.
Barrett, T. J., Green, J. H., Griffin, P. M., Pavia, A. T. Ostroff, S. M., Wachsmuth, I. K. Enzyme-linked immunosorbent assays for detecting antibodies to Shiga-like toxin I, Shiga-like toxin II, andEscherichia coli O157: H7 lipopolysaccharide in human serum. Curr. Microbiol. 23 (1991) 189–195.
Ashkenazi, S., Cleary, T. G., Lopez, E., Pickering, L. K. Anticytotoxin-neutralizing antibodies in immune globulin preparations: Potential use in hemolytic-uremic syndrome. J. Pediatr. 113 (1988) 1008–1014.
Ullman, M. D., McCluer, R. H. Quantitative analysis of plasma neutral glycosphingolipids by high performance liquid chromatography of their perpenzoyl derivatives. J. Lipid Res. 18 (1977) 371–378.
Karmali, M. A., Petric, M., Lim, C., Fleming, P. C., Arbus, G. S. Lior, H. The association between hemolytic uremic syndrome and infection by verotoxin-producingEscherichia coli. J. Infect. Dis. 151 (1985) 775–782.
Sheth, K. J., Gill, J. C., Leichter, H. E. High-dose intravenous gammaglobulin infusions in hemolytic-uremic syndrome: A preliminary report. Am. J. Dis. Child. 144 (1990) 268–270.
Robson, W. L. M., Fick, G. H., Jadavji, T., Leung A. K. C. The use of intravenous gammaglobulins in the treatment of typical hemolytic uremic syndrome. Pediatr. Nephrol. 5 (1991) 289–292.
Scotland, S. M., Rowe, B., Smith, H. R., Willshaw, G., Gross, R. J. Verocytotoxin-producing strains ofEscherichia coli from children with haemolytic uraemic syndrome and their detection by specific DNA probes. J. Med. Microbiol. 25 (1988) 237–243.
Bitzan, M., Moebius, E., Ludwig, K., Müller-Wiefel, D. E., Heesemann, J. High incidence of serum antibodies to antibodies toEscherichia coli O157 lipopolysaccharide in children with hemolytic-uremic syndrome. J. Pediatr. 119 (1991) 380–385.
Sandvig, K., Olsnes, S., Brown, J. E., Petersen, O. W., Deurs, B. v. Endocytosis from coated pits of Shiga toxin: A glycolipid-binding protein fromShigella dysenteriae 1. J. Cell Biol. 108 (1989) 1331–1343.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bitzan, M., Klemt, M., Steffens, R. et al. Differences in verotoxin neutralizing activity of therapeutic immunoglobulins and sera from healthy controls. Infection 21, 140–145 (1993). https://doi.org/10.1007/BF01710530
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01710530