Summary
The existence of thick, stable water films on silica as described byDerjaguin and Kussakov (1939) has been confirmed in several laboratories. There is much evidence showing that their thicknesses can be substantially accounted for by electrical double layer theory. Such wetting films can be influenced by a third component. For example, they are thinned in the presence of inorganic electrolytes or rendered unstable (or metastable) by prior methylation of the silica surface.
Here, we consider the influence of alkyltrimethyl ammonium bromides on the stability of aqueous films on polished fused silica. In particular, the thickness and stability of aqueous films of C5, C8, C10 and C16 trimethyl ammonium bromides were studied over a range of surfactant concentrations. Contact angles of the TAB solutions on silica were also measured.
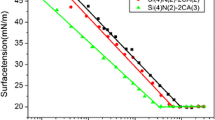
The concentration of each cationic surfactant proved important in determining film stability. In dilute solution (well below the bulk CMC) uniform films could be formed only below a critical concentration which varied with the chainlength of the surfactant. The longer the chainlength, the lower was the critical concentration. In this regime, the film thickness decreased significantly with increasing surfactant concentration. This decrease was greater than that found for a simple 1:1 electrolyte such as KCl at the same concentration. As the surfactant concentration increased towards the critical value, the uniform films became increasingly more prone to rupture.
Once the critical concentration of each surfactant was exceeded, uniform wetting films on silica could not be formed. The films ruptured immediately regardless of their initial diameter. In this regime, the contact angles, measured through the aqueous phase, increased with TAB concentration up to a maximum value which occurred near the CMC.
For C8, C10 and C16 trimethyl ammonium bromides, wetting films were again formed at concentrations above the CMC. At these concentrations, films were studied in detail only for the Cl6 surfactant. In this case, the wetting films were found to be somewhat thicker than free aqueous films of anionic surfactants formed at similar concentrations and hydrostatic pressures.
Zusammenfassung
Die Existenz dicker stabiler Wasserfrlme auf SiO2 (Derjaguin undKussakov (1939)) ist in verschiedenen Laboratorien bestätigt worden. Es gibt zahlreiche Hinweise, nach denen die Dicke durch die elektrische Doppelschichttheorie gedeutet werden kann. Solche benetzenden Filme können durch eine dritte Komponente beeinflußt werden; in Gegenwart anorganischer Elektrolyte werden sie dünner, durch vorhergehende Methylierung der SiO2-Oberfläche instabil oder metastabil.
In der Arbeit wird der Einfluß von Alkyltrimethylammoniumbromiden (C5, C8, C10 und C16 auf die Stabilität von Wasserfilmen auf polierten Quarzglasoberflächen untersucht. Die Konzentration der kationischen Tenside auf die Filmstabilität erwies sich als wichtig. In verdünnten Lösungen bilden sich einheitliche Filme nur unter einer kritischen Konzentration, die von der Kettenlänge des Tensids abhängt. Über der kritischen Konzentration bilden sich keine einheitlich benetzenden Filme; sie reißen sofort unabhängig von ihrem ursprünglichen Durchmesser. Es wird versucht, die Ergebnisse auf der Basis einer Doppelschichttheorie zu deuten.
Similar content being viewed by others
References
Ter-Minassian-Saraga, L., Advan. Chem. Ser.43, 232 (1964).
Elton, G. A. H., Second Intern. Congr. Surface Activity3, 161 (London 1957).
Mingins, J., N. F. Owens, Private communications.
Ruch, R. J., L. S. Bartell, J. Phys. Chem.64, 513 (1960).
Davies, J. T., E. K. Rideal,, Interfacial Phenomena, p. 436 (New York 1961).
Ahrland, S., I. Grenthe, B. Noren, Acta. Chem. Scand.14, 1059 (1960).
Fuerstenau, D. W., J. Phys. Chem.60,981 (1956).
Somasundaren, P., T. W. Healey, D. W. Fuerstenau, J. Phys. Chem.68, 3562 (1964).
Ralston, J., J. A. Kitchener, JCIS50, 242 (1975).
Wark, I. W., J. Phys. Chem.40, 661 (1936).
Manev, E., A. Scheludko, D. Exerwa, Colloid & Polymer Sci.252, 586 (1974).
Blake, T. D., J. A. Kitchener, J. C. S. Faraday I,68, 1435 (1972).
Aronson, M. P., H. M. Princen, JCIS52, 345 (1975).
Bijsterbosch, B. H., JCIS47, 186 (1974).
Mukerjee, P., K. J. Mysels, Critical Micelle Concentrations of Aqueous Surfactant Systems, Nat. Stand. Ref. Data Ser., Nat. Bureau Stand. (Washington 1971).
Blake, T. D., JCS Faraday I,48, 192 (1975).
Scheludko, A., E. Manev, Trans. Faraday Soc.64, 1123 (1968).
Scheludko, A., Private communication.
Derjaguin, B. V., M. Kussakov, Acta Physiochim, USSR10, 153 (1939).
Platikanov, D., J. Phys. Chem.68, 3618 (1964).
Exerwa, D., I. Ivanov, A. Scheludko, Ann. Univ. Sofia Fac. Chem.56, 157 (1961).
Read, A. D., J. A. Kitchener, Soc. Chem. Ind. Monograph25, 300 (1967).
Read, A. D., J. A. Kitchener, JCIS30, 391 (1969).
Derjaguin, B. V., N. V. Churaev, JCIS49, 249 (1974).
Langmuir, I., J. Chem. Phys.6, 873 (1938).
Devereux, O. F., P. L. de Bruyn, Interactions of Plane-Parallel Double Layers (Cambridge, Mass. 1963).
Derjaguin, B. V., L. D. Landau, Zh. Eksp. Teor. Fiz.15, 662 (1945).
Jones, G., L. A. Wood, J. Chem. Phys.13, 106 (1948).
Zorin, Z. M., N. V. Churaev, Colloid J. USSR30, 279 (1968).
Schulze, H. J., Proc. Intern. Conf. Colloid and Surface Science (Budapest) Vol. 1, 179 (1975).
Schulze, H. J. J., Colloid and Polymer Sci.,253, 730 (1975).
Sonntag, H., K. Strenge, Coagulation and Stability of Disperse Systems, p. 94 (New York 1972).
Rubio, J., J. Goldfarb, JCIS36, 289 (1971).
Lyklema, J., K. J. Mysels, J. Am. Chem. Soc.87, 2539 (1965).
Scheludko, A., D. Exerova, Kolloid-Z.168, 24 (1960).
Author information
Authors and Affiliations
Additional information
With 7 figures and 2 tables
Rights and permissions
About this article
Cite this article
Aronson, M.P., Princen, H.M. Aqueous films on silica in the presence of cationic surfactants. Colloid & Polymer Sci 256, 140–149 (1978). https://doi.org/10.1007/BF01679172
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01679172