Abstract
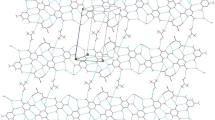
Crystals consisting of two distinct chemical entities, tautomers of each other, in exact 1∶1 ratio, have been obtained and their structure determined by X-ray analysis. The crystals of C9H11N3·C9H11N3 are monoclinic,P21/c,a=15.674(3),b=17.085(3),c=13.758(3)Å, β=90.78(2)°,Z=8. There are two hydroxylamine and two aminonitrone molecules in the asymmetric unit. Hydrogen bonds connect those molecules into chiral layers. Layers of opposite chirality alternate andthe crystal is centrosymmetric as a whole. Within those layers chains of tautomers joined by very strong O−H... O and strong N−H... N bonds can be recognized. Proton transfer along those chains with simultaneous rearrangement of π-bonds within the molecules would result in interconversion of tautomers and would affect chirality of the layer.
Similar content being viewed by others
References
Eliel, E. L.; Wilen, S. H.; Mander, L. N.Stereochemistry of Organic Compounds; Wiley: New York, 1994; p 1209.
Elguero, J.; Marzin, C.; Katritzky, A. R.; Linda P.The Tautomerism of Heterocycles; Academic Press: New York, 1976.
Katritzky, A. R.; Karelson, M.; Harris, P. A.Heterocycles 1991,32, 329.
Sharma, B. D.; McConnell, J. F.Acta Crystallogr. 1965,19, 797.
Webb, L. E.; Fleisher, E. B.J. Chem. Phys. 1965,43, 3100.
Vogel, E.; Kocher, M.; Schmickler, H.; Lex, J.Angew. Chem., Int. Ed. Engl. 1986,25, 257.
Bechtel, F.; Gaultier, J.; Hauw, C.Cryst. Struct. Commun. 1973,2, 469.
Freeman, H. C.; Hutchinson, N. D.Acta Crystallogr. Sect. B 1979,35, 2051.
Amor, A. B. H.; Kallel, A.; Paulus, H.Acta Crystallogr. Sect. C. 1989,45, 782.
Moore, F. H., White, A. H., Willis, A. C.J. Chem. Soc. Perkin Trans. 21975, 1068.
Toda, F.; Tanaka, K.; Elguero, J.; Stein, Z.; Goldberg, I.Chem. Lett. 1988, 1061.
Dupont, L.; Dive, G.Bull. Soc. R. Sci. Liege 1982,51, 248.
Weiss, A. L.; Frolow, F.; Vishkautsan, R.J. Heterocycl. Chem. 1986,23, 705.
Ratilla, E. M. A.; Scott, B. K.; Moxness, M. S.; Kostic, N. M.Inorg. Chem. 1990,29, 918.
Hingerty, B. E.; Einstein, J. R.; Wei, C. H.Acta Crystallogr. Sect. B 1981,37, 140.
Whinnery, J. E.; Watson, W. H.Acta Crystallogr. Sect. B 1972,28, 3635.
Barkigia, K. M.; Fajer, J.; Chang, C. K.; Williams, G. J. B.J. Am Chem. Soc. 1982,104, 315.
Saczewski, F.; Debowski, T.Tetrahedron Lett. 1993,34, 2843.
Branco, P. S.; Prabhakar, S.; Lobo, A. M.; Williams, D. J.Tetrahedron 1992,48, 6335.
Jackman, L. M.; Jen, T.J. Am. Chem. Soc. 1975,97, 2811.
Taylor, E. C.J. Org. Chem. 1971,36, 634.
Lehmann, M. S.; Larsen, F. K.Acta Crystallogr., Sect. A 1974,30, 580.
Sheldrick, G. M.SHELXS86. Program for the Solution of Crystal Structures Univ. of Göttingen, Germany,1986.
Sheldrick, G. M.SHELXL93. Program for the Refinement of Crystal Structures. Univ. of Göttingen, Germany,1993.
Etter, M. C.; MacDonald, J. C.; Bernstein J.Acta Crystallogr., Sect. B 1990,46, 256.
Jeffrey, G. A.; Saenger, W.Hydrogen Bonding in Biological Structures. Springer-Verlag: Berlin, Heilderberg, 1991; pp 35–37.
Allen, F. H.; Kennard, O.; Taylor R.Chemical Design Automation News 1993,8, 131.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gdaniec, M., Sączewski, F. & Dębowski, T. α-Iminohydroxylamine-α-aminonitrone tautomerism: a 1∶1 mixture of two tautomeric forms in crystals of N-(4,5-dihydro-1H-imidazol-2-yl)-N-phenyl-hydroxylamine. J Chem Crystallogr 25, 813–821 (1995). https://doi.org/10.1007/BF01671076
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01671076