Summary
The yield of phosphorus in sorghum plants grown on samples of 22 different soils from the United States was determined in the greenhouse. Analyses of variance of the regressions of these values on the measurements of soil phosphorus by various laboratory methods were calculated as an aid in evaluating the methods.
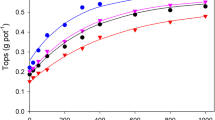
No general advantage was found from incubating the samples in moist condition for a week before analysis versus making the analysis on the initially-dry samples. In the order of decreasing precision of predicting the yields of phosphorus the methods are as follows: (1) anion-exchange resin method of Amer and coworkers, (2) 0.5M NaHCO3 method of Olsen and coworkers, (3) phosphate potential method of Schofield and Aslyng, (4) phosphorus concentration in the 0.01M CaCl2 extract of Schofield and Aslyng, and (5) 0.1N HCl, 0.03N NH4F method of Bray and Kurtz.
After taking into account the additional variables, little or no improvement in precision of prediction was obtained when the phosphorussorbedvs. time curves for the anion-exchange-resin method were (a) divided into four segments on the basis of time, the quantities of phosphorus in the four segments being used as independent variables in a multiple-regression equation, or (b) broken down into a maximum of four components on the assumption that the overall curve represents the summation of a group of simultaneous first-order reactions, the quantities of phosphorus in the several components being used as independent variables in a multiple-regression equation.
The precision of prediction was improved by using as the soil-phosphorus measurement the sum of the products of the rate of phosphorus extraction by the anion-exchange-resin method and the quantities of phosphorus extracted within individual time intervals. A logarithmic expression was used to fit the relationship, however, and it appeared that the greater precision of prediction resulted from the logarithmic transformation rather than the superiority of the method as such.
The precision of prediction was improved also by using the H2PO −4 concentration instead of the total-inorganic-phosphorus concentration as the independent variable in the 0.01M CaCl2 extracts of Schofield and Aslyng and by using the H2PO −4 instead of the total inorganic phosphorus sorbed by the anion-exchange resin. This modification made the anion-exchange-resin method considerably better than the others tested.
Similar content being viewed by others
Literature cited
Amer, F., Bouldin, D. R., Black, C. A., and Duke, F. R., Characterization of soil phosphorus by anion exchange resin adsorption and P32-equilibration. Plant and Soil6, 391–408 (1955).
Aslyng, H. C., The lime and phosphate potentials of soils; the solubility and availability of phosphates. Kgl. Vet. og Landbohøjskole Års.1954, 1–50 (1954).
Bouyoucos, George John, A comparison between the suction method and the centrifuge method for determining the moisture equivalent of soils. Soil Sci.40, 165–171 (1935).
Bray, Roger H., and Kurtz, L. T., Determination of total, organic, and available forms of phosphorus in soils. Soil Sci.59, 39–45 (1945).
Dickman, S. R., and Bray, R. H., Colorimetric determination of phosphate. Ind. Eng. Chem. Anal. Ed.12, 665–668 (1940).
Fried, Maurice, and Dean, L. A., A concept concerning the measurement of available soil nutrients. Soil Sci.73, 263–271 (1952).
Olsen, Sterling R., Cole, C. V., Watanabe, Frank S., and Dean, L. A., Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Dept. Agr. Circ.939 (1954).
Schofield, R. K., The equilibrium concentration of phosphate in soil solutions.In: Plant and Animal Nutrition in Relation to Soil and Climatic Factors. Proc. Specialist Conf. Agr. Australia 1949 (p. 124). H.M.S.O., London (1951).
Additional information
Journal Paper No. J-3452 of the Iowa Agricultural and Home Economics Experiment Station, Ames, Iowa. Project No. 1183. Contribution from the Department of Agronomy.
Graduate Students and Professor of Soils, respectively.
Rights and permissions
About this article
Cite this article
Moser, U.S., Sutherland, W.H. & Black, C.A. Evaluation of laboratory indexes of absorption of soil phosphorus by plants: I. Plant Soil 10, 356–374 (1959). https://doi.org/10.1007/BF01666210
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01666210